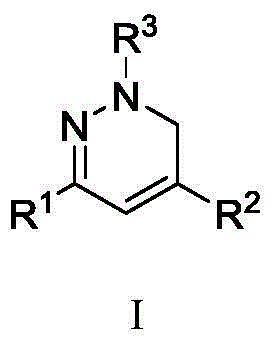
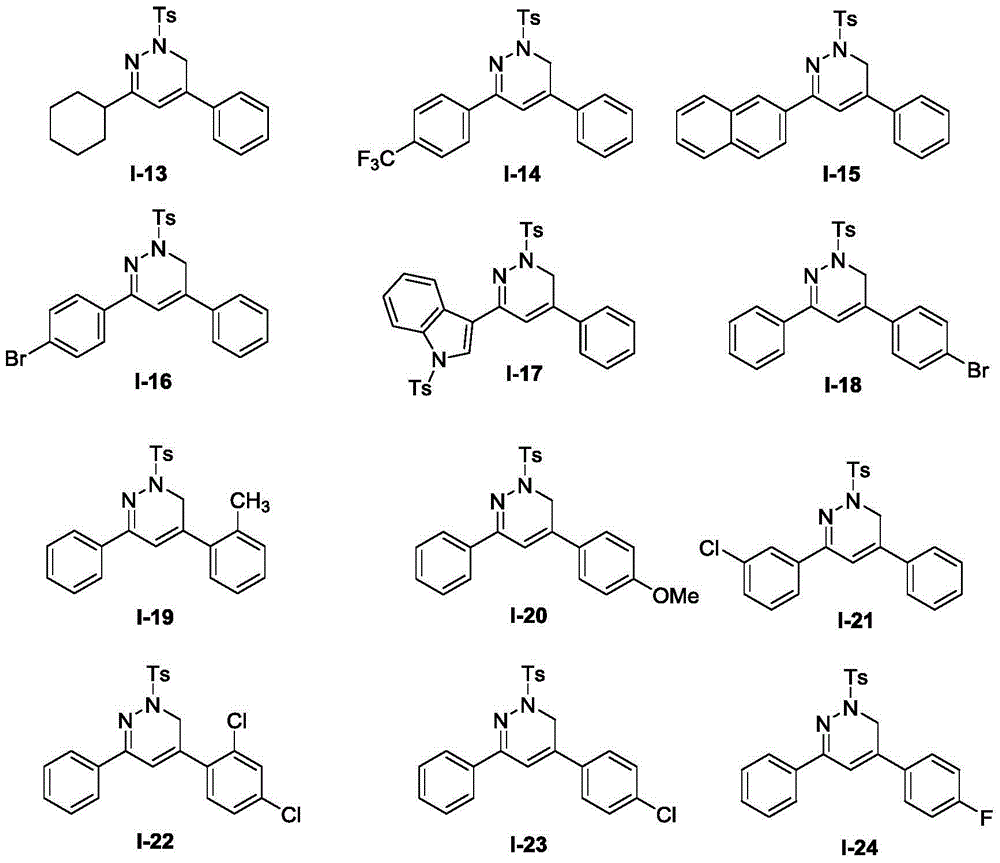
Synthetic method and application of 1, 6-dihydro pyridazine and pyridazine compounds in inhibition of growth activity of five common pathogenic fungis
Technology of a compound, pyridazine salt, applied in the field of chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
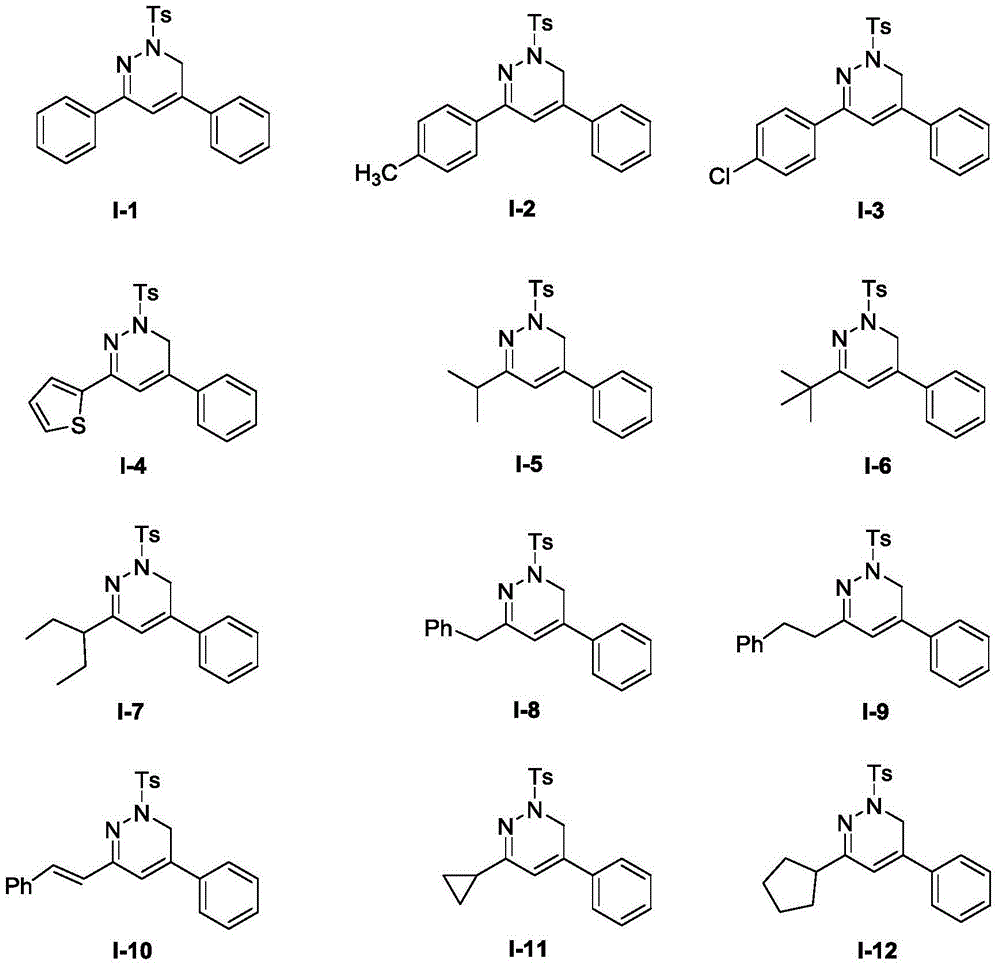
[0061] Compound I-1
[0062]
[0063] At room temperature, starting material β, γ-unsaturated hydrazone A-1 (117.0mg, 0.3mmol), 1.0 equivalent of 2,2,6,6-tetramethylpiperidine oxide (46.9mg, 0.3mmol), 1.5 Equivalent inorganic base potassium carbonate (62.2mg, 0.3mmol) and 2mol% photocatalyst Ru(bpy) 3 Cl 2 ·6H 2 O (4.5mg, 0.006mmol) was dissolved in anhydrous chloroform, and the trace oxygen in the solvent was removed under the protection of argon. Then react at room temperature under the irradiation condition of the blue LED lamp of 3W until TLC detects that the reaction is complete, with V 石油醚 / V 乙酸乙酯 =20:1-10:1 column chromatography directly obtains the target product of formula I-1 with a yield of 84%.
[0064] 1 H NMR (600MHz, CDCl 3 )δ(ppm)δ=7.95(d,J=8.2Hz,2H),7.81–7.81(m,2H),7.57(d,J=7.0Hz,2H),7.47–7.44(m,3H),7.43– 7.42 (m, 3H), 7.38 (d, J=8.1Hz, 2H), 6.81 (s, 1H), 4.36 (s, 2H), 2.45 (s, 3H).
[0065] 13 C NMR (100MHz, CDCl 3 ) δ (ppm) δ = 150.71, 144.27, ...
Embodiment 2
[0069] Compound I-2
[0070]
[0071] At room temperature, starting material β, γ-unsaturated hydrazone A-2 (121.2mg, 0.3mmol), 1.0 equivalent of 2,2,6,6-tetramethylpiperidine oxide (46.9mg, 0.3mmol), 1.5 An equivalent amount of inorganic base potassium carbonate (62.2mg, 0.3mmol) and 2mol% photocatalyst (4.5mg, 0.006mmol) were dissolved in anhydrous chloroform, and trace oxygen in the solvent was removed under the protection of argon. Then react at room temperature under the irradiation condition of a blue LED light of 3W until the TLC detection reaction is complete, directly obtain the target product of formula I-2 with V petroleum ether / V ethyl acetate=20:1-10:1 column chromatography, product The rate is 85%. 1 H NMR (600MHz, CDCl 3 )δ(ppm)δ=7.91(d,J=8.1Hz,2H),7.66(d,J=8.0Hz,2H),7.52(d,J=7.7Hz,2H),7.40–7.33(m,3H ),7.33(d,J=8.0Hz,2H),7.20(d,J=8.0Hz,2H),6.76(s,1H),4.30(s,2H),2.40(s,3H),2.37(s ,3H).
[0072] 13 C NMR (100MHz, CDCl 3 ) δ (ppm) δ = 150.77, 144.26, 139....
Embodiment 3
[0075] Compound I-3
[0076]
[0077] At room temperature, starting material β, γ-unsaturated hydrazone A-3 (127.2mg, 0.3mmol), 1.0 equivalent of 2,2,6,6-tetramethylpiperidine oxide (46.9mg, 0.3mmol), 1.5 An equivalent amount of inorganic base potassium carbonate (62.2mg, 0.3mmol) and 2mol% photocatalyst (4.5mg, 0.006mmol) were dissolved in anhydrous chloroform, and trace oxygen in the solvent was removed under the protection of argon. Then react at room temperature under the irradiation conditions of a 3W blue LED lamp until the TLC detection reaction is complete, and directly obtain the target product of formula I-3 with V petroleum ether / V ethyl acetate=20:1-10:1 column chromatography, producing The rate is 74%.
[0078] 1 H NMR (600MHz, CDCl3) δ (ppm) δ = 7.93 (d, J = 7.9Hz, 2H), 7.73 (d, J = 8.2Hz, 2H), 7.56 (d, J = 6.7Hz, 2H), 7.48 -7.45 (m, 3H), 7.39 (t, J=9.1Hz, 4H), 4.36 (s, 2H), 2.45 (s, 3H).
[0079] 13 C NMR (100MHz, CDCl3) δ(ppm) δ=149.67, 144.42, 140.09, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com