Method of preparing 1,5-amino alcohol
An amino alcohol and phenyl technology, applied in the field of preparation 1, can solve the problems of harsh experimental conditions, cumbersome raw material synthesis, expensive reagents, etc., and achieves the effects of mild reaction conditions, cheap and easily available reagents, and large industrialization potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
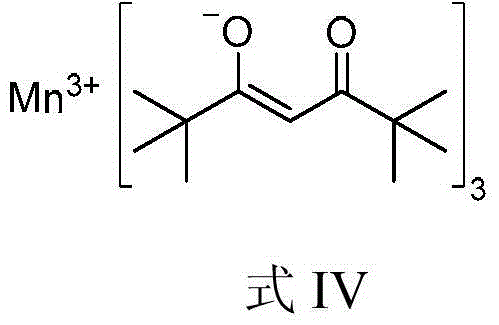
Method used
Image
Examples
Embodiment 1
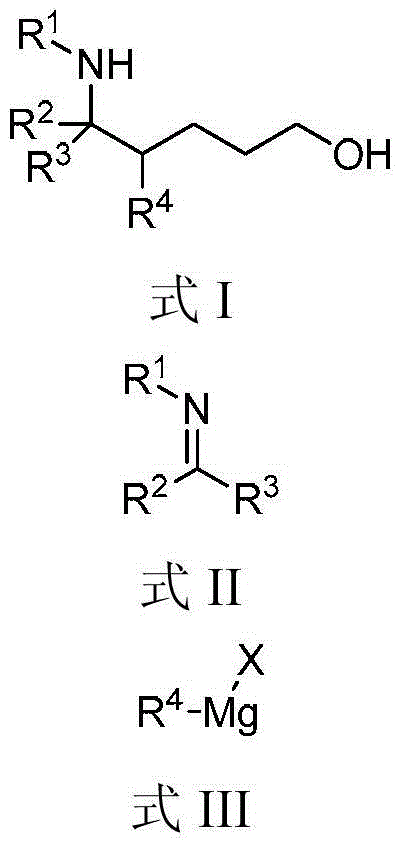
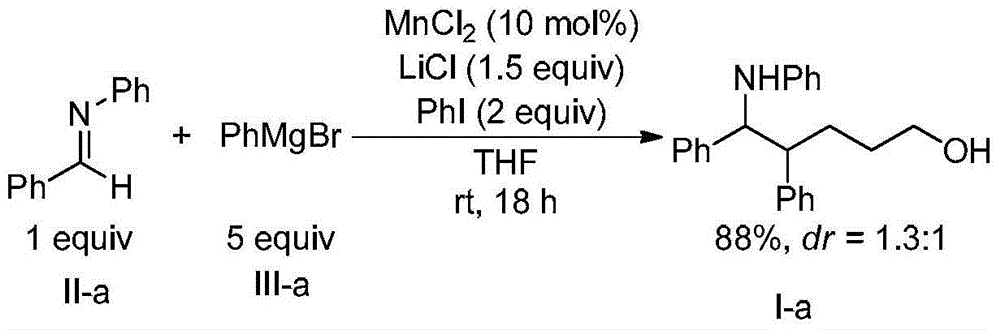
[0025] Example 1, 4,5-diphenyl-5-(phenylamino)-1-pentanol (formula Ⅰ-a)
[0026]
[0027] Add N-benzylideneaniline (Formula II-a) (2mmol, 362mg), catalyst manganese dichloride (0.2mmol, 25mg), additive lithium chloride (3mmmol, 127mg), oxidant iodobenzene ( 4mmol, 816mg), solvent tetrahydrofuran (5mL) and phenylmagnesium bromide (formula III-a) (tetrahydrofuran solution, 1mol / L) (10mmol, 10mL), three-component reaction at room temperature. After 18h with saturated NaHCO 3 Solution (40mL) was used to quench the reaction, and then extracted with dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate, filtered, and spin-dried. 1 The diastereomeric ratio of 1,5-aminoalcohol (Formula I-a) in the crude product was detected by H NMR to be 1.3:1. The crude product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 10 / 1, v / v) to obtain 583 mg of the target product (Formula I-a), with a yield of 88%.
[0028] The major d...
Embodiment 2
[0030] Example 2, 4-phenyl-5-(methylthiophenyl)-5-(phenylamino)-1-pentanol (formula Ⅰ-b)
[0031]
[0032] Add imine (formula II-b) (2mmol, 454mg), catalyst manganese diacetate (0.1mmol, 17mg), lithium chloride (3mmmol, 127mg), iodobenzene (3mmol, 612mg), tetrahydrofuran ( 9.3mL) and phenylmagnesium chloride (formula III-b) (tetrahydrofuran solution, 1.4mol / L) (8mmol, 5.7mL) at 0°C for a three-component reaction. After 24 h with saturated NaHCO 3 Solution (40mL) was used to quench the reaction, and then extracted with dichloromethane, and the organic phases were combined, dried with anhydrous sodium sulfate, filtered, and spin-dried. 1 The diastereomeric ratio of 1,5-aminoalcohol (Formula I-b) in the crude product was 1.4:1 by H NMR. The crude product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 10 / 1, v / v) to obtain 718 mg of the target product (Formula I-b), with a yield of 95%.
[0033] The major diastereomers are characterized as fol...
Embodiment 3
[0035] Example 3, 4-phenyl-5-(4-chlorophenyl)-5-((2,4,6-trimethylphenyl)amino)-1-pentanol (Formula I-c)
[0036]
[0037] Add imine (Formula II-c) (2mmol, 516mg), catalyst manganese diacetylacetonate (0.1mmol, 25mg), lithium chloride (3mmmol, 127mg), iodobenzene (3mmol, 612mg), tetrahydrofuran into a 50mL flask in sequence (9.3mL) and phenylmagnesium chloride (formula III-b) (tetrahydrofuran solution, 1.4mol / L) (8mmol, 5.7mL) at 40°C for a three-component reaction. After 18h with saturated NaHCO 3 Solution (40mL) was used to quench the reaction, and then extracted with dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate, filtered, and spin-dried. 1 The diastereomeric ratio of 1,5-aminoalcohol (Formula I-c) in the crude product was determined to be 3.3:1 by H NMR. The crude product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 10 / 1, v / v) to obtain 775 mg of the target product (Formula I-c), with a yield o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com