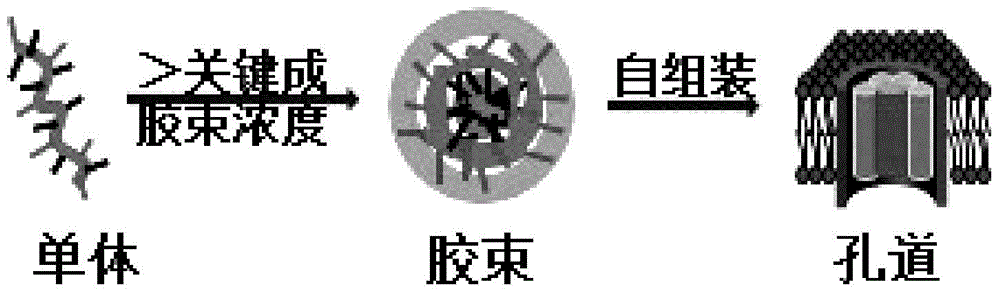
Cation amphiphilic membrane targeted alpha-helix polypeptides and application thereof
An amphiphilic membrane-targeted, cationic technology, applied in the direction of peptides, peptide/protein components, preparations for in vivo tests, etc., can solve problems such as difficult large-scale application, low yield, and complicated operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] When X 1 is Arg, X 2 for Leu, X 3 When it is Ala, the polypeptide sequence (SEQ ID No.2, also referred to as sequence 2) is as follows: HGG-(RLARLAR) 2 -NH 2 .
[0044] 1. Key micelle concentration experiment of peptides
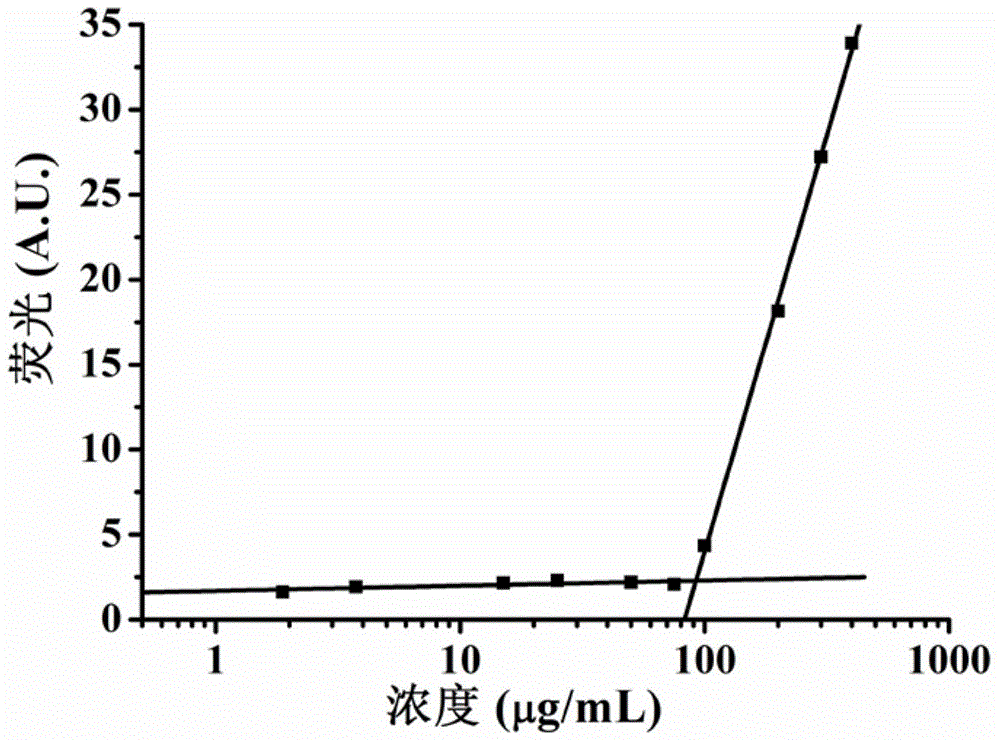
[0045] In the key micelle concentration experiment of the peptide, the peptide was diluted with Milli Q water, the concentration range was 1.56-300 μg / ml, and Nile Red was dissolved in dichloromethane to form a 500 micromolar solution, and 10 Microliter Nile Red, keep away from light to make dichloromethane evaporate to dryness, add 500 microliters of peptide solutions of various concentrations to the centrifuge tube containing Nile Red, keep away from light at 25 oC, shake overnight in a shaker at 250 rpm, use 485 nm excitation, fluorescence spectrometer to measure the fluorescence intensity of Nile Red at each concentration. Figure 2A The key micelle concentration curve of polypeptide sequence 2 is shown.
[0046] 2. Membrane-targeted local...
Embodiment 2
[0049] When X 1 is Arg, X 2 for Leu, X 3 When it is Leu, the polypeptide sequence (SEQ ID No.5, also referred to as sequence 5) is as follows: HGG-(RLRLLR) 2 -NH 2 .
[0050] 1. The key micelle concentration experiment of polypeptide
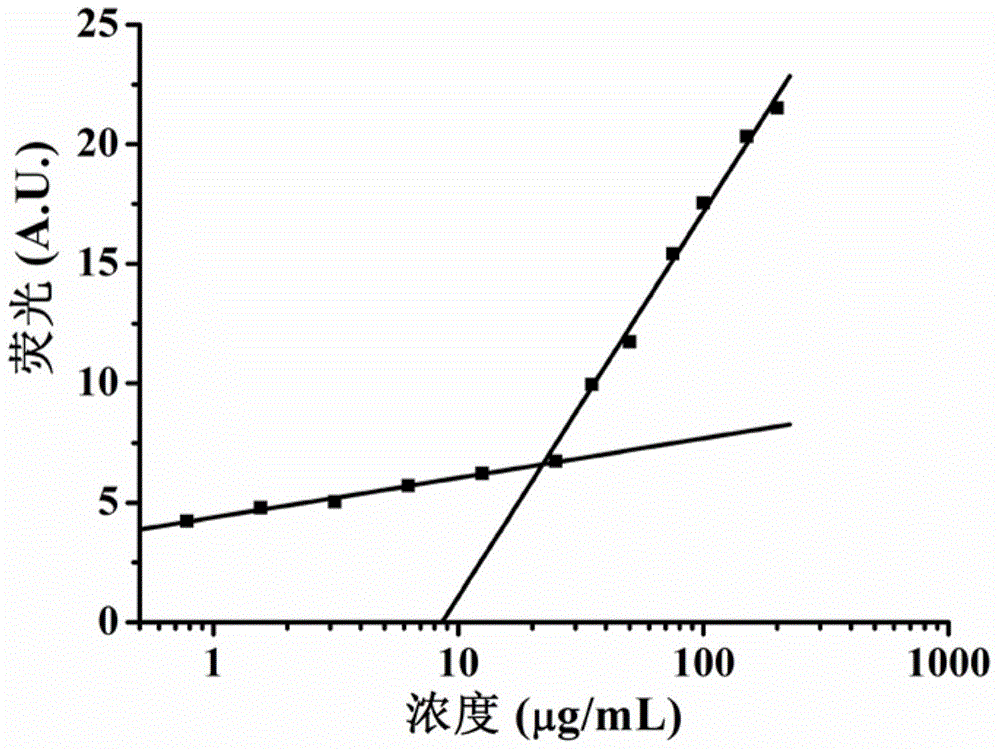
[0051] In the key micelle concentration experiment of the peptide, the peptide was diluted with Milli Q water, the concentration range was 1.56-300 μg / ml, and Nile Red was dissolved in dichloromethane to form a 500 micromolar solution, and 10 Microliter Nile Red, keep away from light to make dichloromethane evaporate to dryness, add 500 microliters of peptide solutions of various concentrations to the centrifuge tube containing Nile Red, keep away from light at 25 oC, shake overnight in a shaker at 250 rpm, use 485 nm excitation, fluorescence spectrometer to measure the fluorescence intensity of Nile Red at each concentration. Figure 2B The concentration change curve of the key micelle concentration of polypeptide sequence 5 is shown.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com