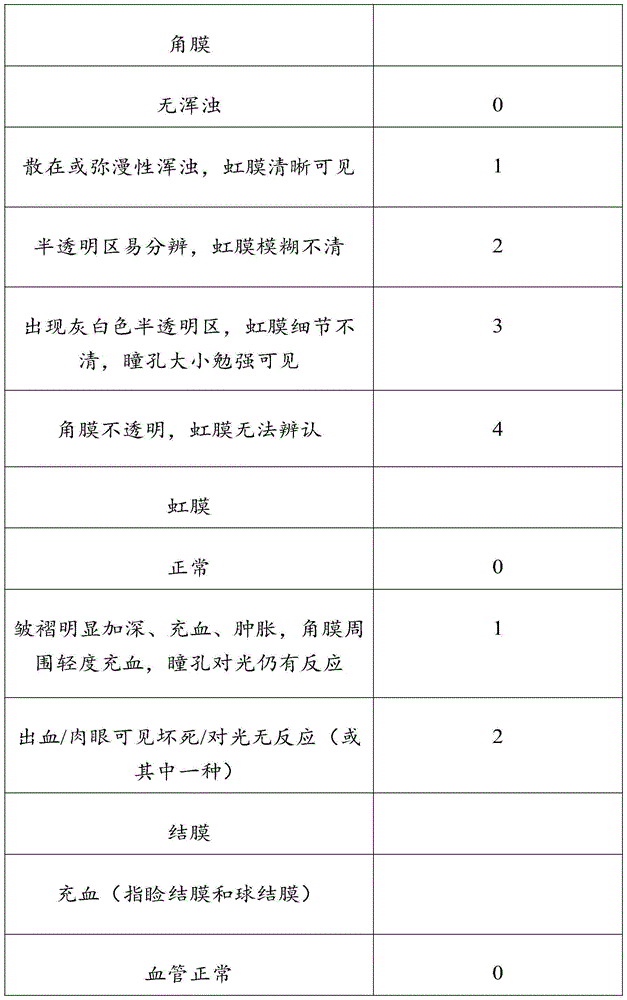
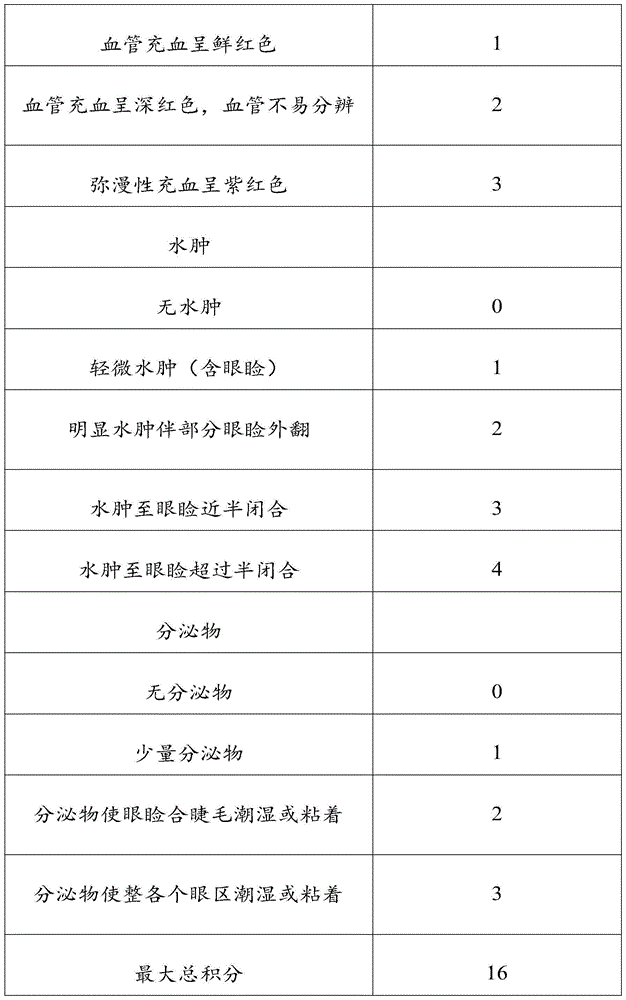
Recombinant human basic fibroblast growth factor gel without bacteriostatic agent and preparation method thereof
A fibroblast and growth factor technology, applied in the field of recombinant human basic fibroblast growth factor gel and its preparation, can solve problems such as adverse reactions and achieve the effect of avoiding stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The embodiment of the present invention provides a recombinant human basic fibroblast growth factor gel that does not contain bacteriostatic agents, including the following components: recombinant human basic fibroblast growth factor 1×10 6 IU, mannitol 0.01g, polyvinyl alcohol 0.1g, sodium hydroxide 0.1g, phosphate buffered saline 0.1g, glycerol 0.1g, add water for injection to make up to 1000ml.
[0023] The embodiment of the present invention also provides a preparation method of recombinant human basic fibroblast growth factor gel without bacteriostatic agent, comprising the following steps:
[0024] S1: Weigh 0.1 g of polyvinyl alcohol, dissolve 0.1 g of sodium hydroxide in water for injection, and sterilize by autoclaving at 121°C for 30 minutes;
[0025] S2: Weigh recombinant human basic fibroblast growth factor 1×10 6 IU, mannitol 0.01g, phosphate buffer 0.1g, glycerin 0.1g were dissolved in water for injection;
[0026] S3: Filter the medicinal solution obtai...
Embodiment 2
[0029] The embodiment of the present invention provides a recombinant human basic fibroblast growth factor gel that does not contain bacteriostatic agents, including the following components: recombinant human basic fibroblast growth factor 9×10 6 IU, sodium lauryl sulfate 5.0g, carboxymethylcellulose 20.0g, sodium carbonate 10.0g, sodium citrate / citrate buffer pair 10.0g, propylene glycol 50g, add water for injection to make up to 1000ml.
[0030] The preparation method of the bacteriostatic-free recombinant human basic fibroblast growth factor gel provided in this example is the same as that in Example 1.
Embodiment 3
[0032] The embodiment of the present invention provides a recombinant human basic fibroblast growth factor gel that does not contain bacteriostatic agents, including the following components: recombinant human basic fibroblast growth factor 6×10 6 IU, hyaluronic acid 1g, sodium alginate 3g, disodium hydrogen phosphate 2.5g, phosphate buffered saline 1.5g, glycerin 30g, add water for injection to make up to 1000ml.
[0033] The preparation method of the bacteriostatic-free recombinant human basic fibroblast growth factor gel provided in this example is the same as that in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Growth factor | aaaaa | aaaaa |
| Growth factor | aaaaa | aaaaa |
| Growth factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com