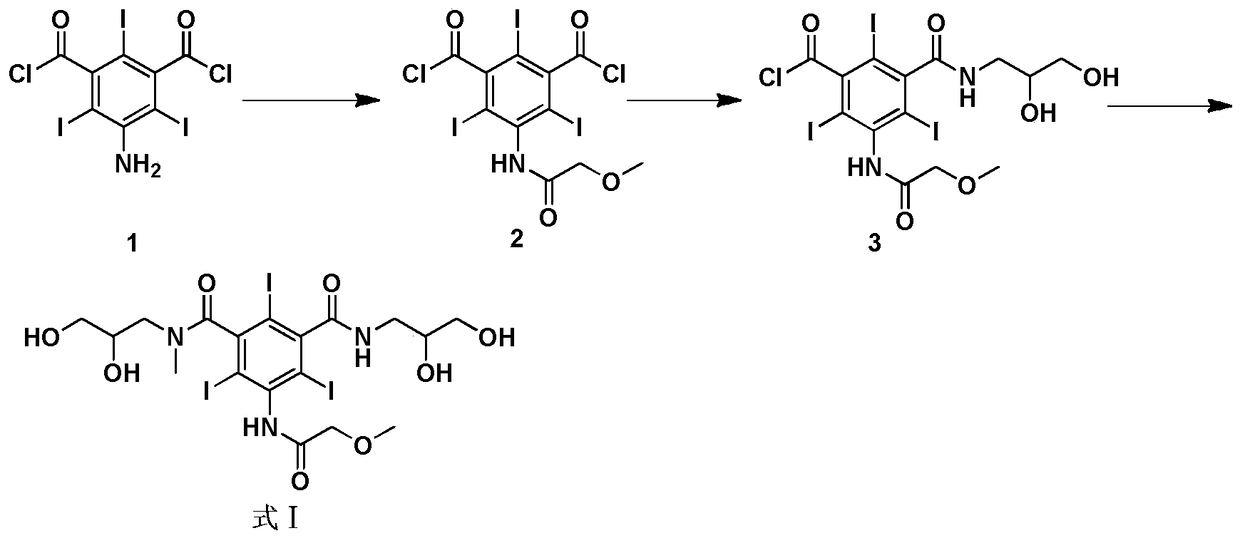
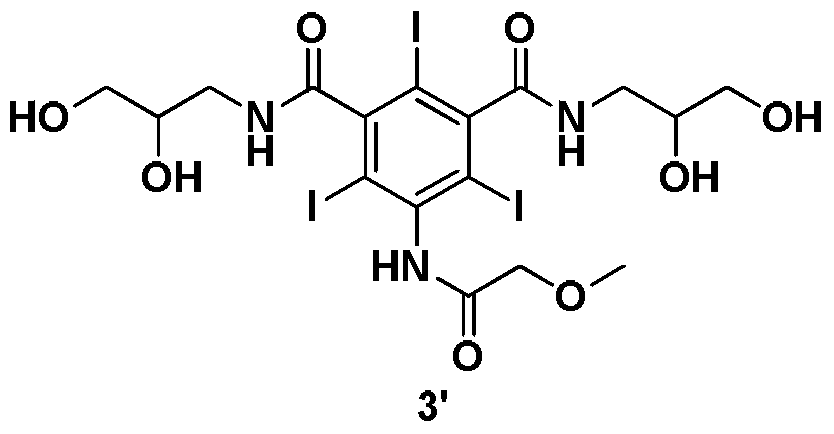
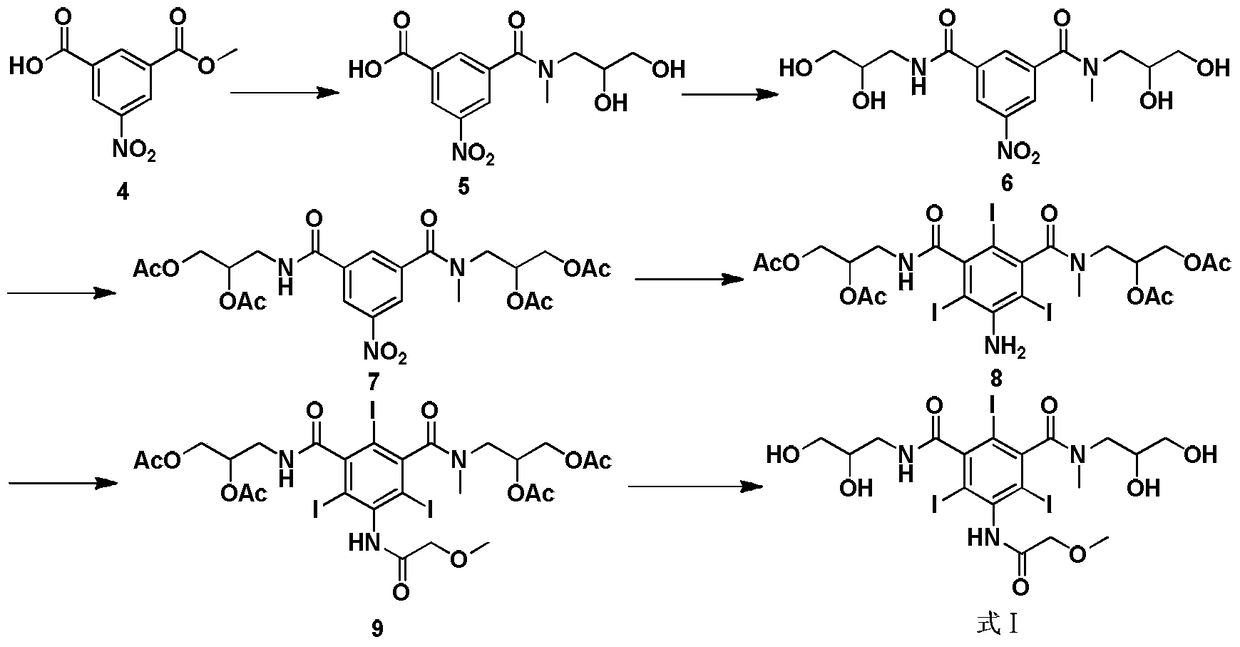
New method for preparing iopromide
A technology of iopromide and triiodobenzoyl chloride, which is applied in the field of preparation of iopromide, can solve the problems of reducing the polarity of compounds, not easy to remove protecting groups, and many reaction steps, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of 5-amino-3-allylcarbamoyl-2,4,6-triiodobenzoyl chloride (formula III):
[0042] Method 1: Dissolve 5-amino-2,4,6-triiodoisophthalic acid dichloride (Formula II) (100.0g, 0.17mol) in 500mlTHF, cool down in an ice-salt bath to below 0°C, add dropwise 500ml of allylamine (30.5g, 0.53mol) solution dissolved in THF was added after dropping, and reacted below 0°C for 10h. After the reaction was completed, suction filtered, and the filtrate was evaporated to dryness to obtain a light yellow white powder. The crude product was slurried with dichloromethane, filtered with suction, and the filtrate was evaporated to dryness to obtain 62.1 g of beige powder with a molar yield of 60%.
[0043] Method 2: N,N-dimethylformamide was used as the reaction solvent, allylamine (10.96g, 0.19mol) was operated as above, and the reaction was carried out at room temperature for 8 hours, and the product yield was 61%.
[0044] Method 2: Using N,N-dimethylformamide as the ...
Embodiment 2
[0045] Example 2: Synthesis of 5-methoxyacetamido-2,4,6-triiodoisophthaloyl chloride (Formula III-1):
[0046] Dissolve methoxyacetyl chloride (54.6g, 0.50mol) in 100mL of dry DMA, add dropwise 5-amino-2,4,6-triiodoisophthalic acid dichloride (Formula II) at 10°C (100.0 g, 0.17 mol) of DMA solution in 300 mL, after dropping, react at room temperature for 24 hours. After the reaction is completed, the reaction solution is poured into ice water, and a white solid is precipitated immediately, stirred for 15 min, filtered with suction, and the filter cake is dissolved in dichloromethane, and then washed with saturated NaHCO 3 solution and saturated NaCl solution were washed twice, and the organic layer was placed in the refrigerator with a small amount of anhydrous NaCl 2 SO 4 After drying overnight and removing the solvent, 101.1 g of a white solid was obtained, and the molar yield was 90.2%.
[0047] Example: 3: Synthesis of 5-amino-N-methyl-N, N'-diallyl-2,4,6-triiodoisophth...
Embodiment 4
[0051] Example 4: Preparation of 5-methoxyacetamido-3-allylcarbamoyl-2,4,6-triiodobenzoyl chloride (Formula IV-1):
[0052] Method 1: At room temperature, dissolve methoxyacetyl chloride (31.7g, 0.29mol) in 300mlTHF, and add it dropwise to 5-amino-3-allylcarbamoyl-2,4,6-triiodobenzene Formyl chloride (formula III) (60.0 g, 0.10 mol) in 300 ml THF was added dropwise and reacted at room temperature. After the reaction, evaporate to dryness under reduced pressure to obtain 65.0 g of white powder with a molar yield of 97%.
[0053] Method 2: Using N,N-dimethylformamide as the reaction solvent, the operation is the same as above, and the product yield is 95%.
[0054] Method 2: Using N,N-dimethylformamide as the reaction solvent, the operation is the same as above, and the product yield is 97%.
[0055] Method 4: Use acetonitrile as the reaction solvent, add triethylamine (29.0 g, 0.29 mol), after the reaction is completed, filter with suction, and evaporate the reaction liquid to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com