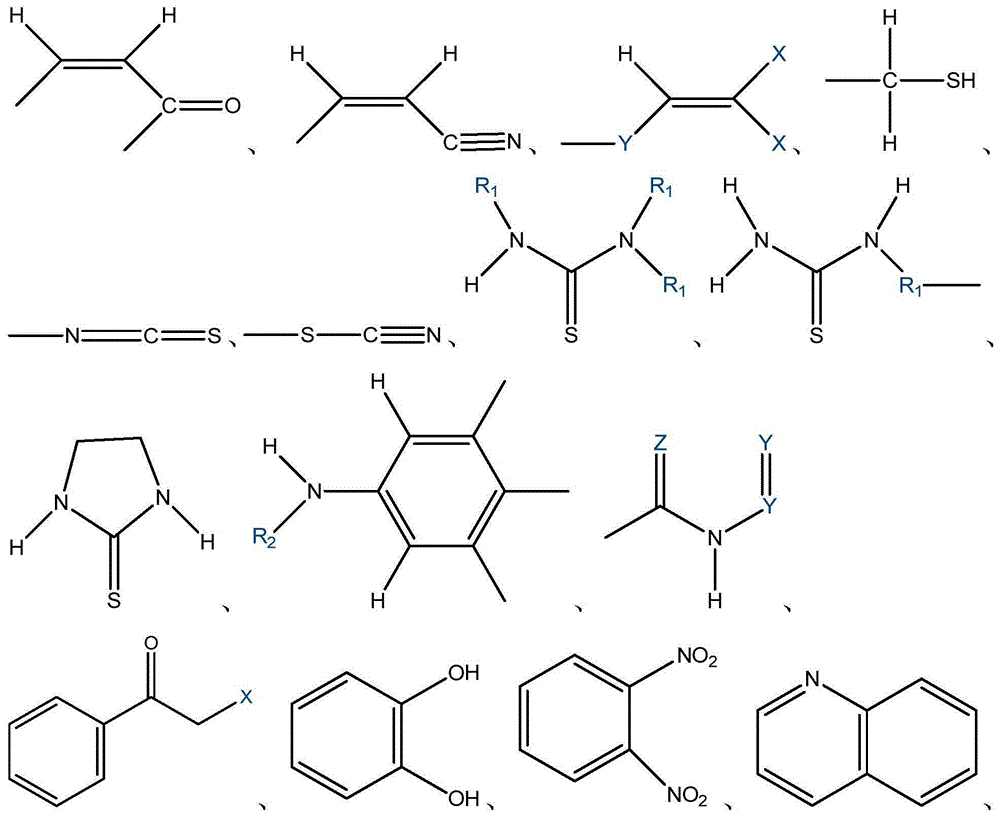
QSAR (Quantitative Structure-Activity Relationships) model constructed based on comprehensive toxicity action mode classification for predicting acute toxicity of organic compound to daphnia magna
A technology of organic compounds and toxic effects, applied in character and pattern recognition, computer components, special data processing applications, etc., can solve problems such as limited model development, complex MOA classification, and less research on reactive compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
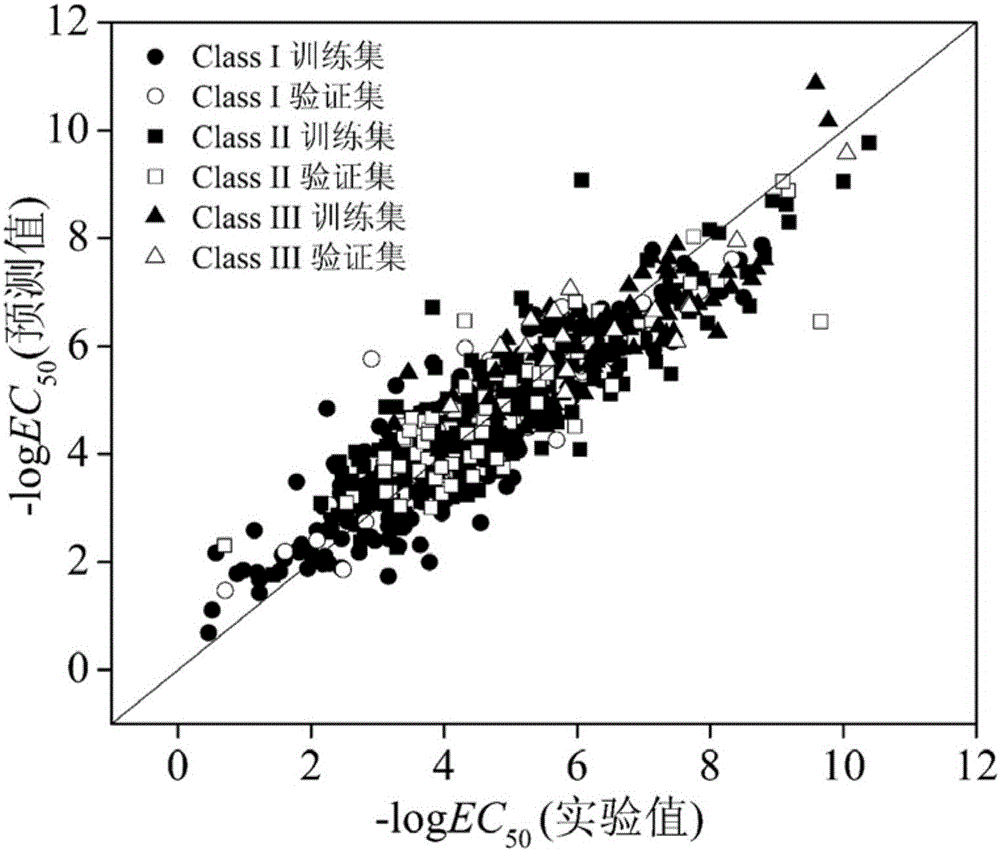
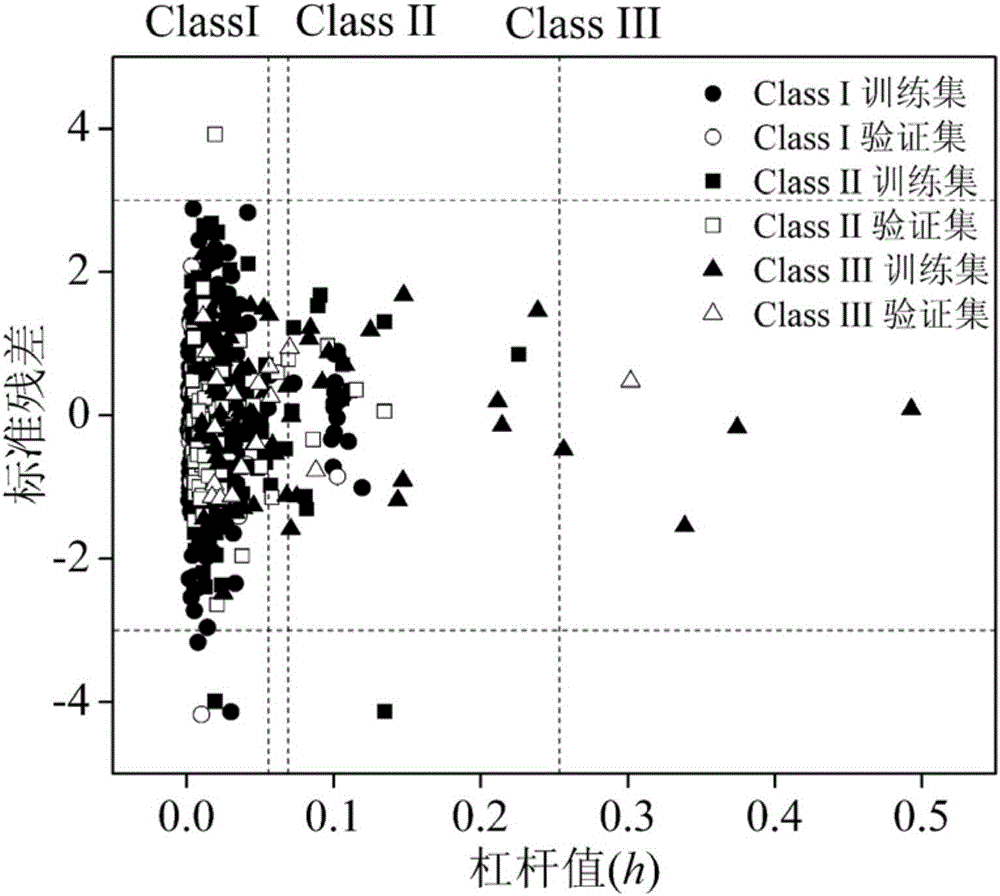
[0053] Given a compound, 1,2-dichloropropane (CAS No. 78-87-5), predict its acute toxicity value for Daphnia magna. Firstly, it is classified according to the comprehensive toxicity mode classification rules proposed by the present invention, which conforms to rule 3 and belongs to class I. The molecular structure of the compound was optimized. Based on the optimized molecular structure, the values of MLOGP, X3sol, nArCOOH, O-056 and GATS1s were calculated to be 2.226, 1.531, 0, 0, and 0.628, respectively, using Draogon6.0 software. Then the h value calculated according to the formula (1) is 0.0034 (50 The value is 3.63, of which the experimental value is 3.58, and the prediction result is good.
Embodiment 2
[0055]Given a compound acetaldehyde (CAS No. 75-07-0), predict its acute toxicity value for Daphnia magna. Firstly, it is classified according to the classification rules, which conforms to rule 2.2 and belongs to class II. The molecular structure of the compound was optimized. Based on the optimized molecular structure, the values of MLOGP2, D / Dtr03, Ks, GATS1p, F08[C-C], and SAdon were calculated as 0.101, 0, 0.701, 1.74, 0, and 0, respectively, using Draogon 6.0 software. Then the h value calculated according to the formula (1) is 0.0199 (50 The value is 3.22, of which the experimental value is 3.17, and the prediction result is good.
Embodiment 3
[0057] Given a compound trichlorfon (CAS No. 52-68-6), predict its acute toxicity value for Daphnia magna. Firstly, it is classified according to the classification rules, which conforms to rule 1.1 and belongs to class III. The molecular structure of the compound was optimized. Based on the optimized molecular structure, the values of RDF115m, GATS5s, GGI5, CATS2D_04_DA, and Psi_i_0d were calculated as 0, 0.829, 0, 0, and 0.003, respectively, using Draogon 6.0 software. Then the h value calculated according to the formula (1) is 0.0379 (50 The value is 6.30, of which the experimental value is 6.31, and the prediction result is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com