Trifluoromethyl containing quinazoline derivative and preparation method and application thereof
A quinazolinone, trifluoromethyl technology, applied in the field of medicinal chemistry, can solve the problems of harsh reaction conditions, low yield, change, etc., and achieve the effects of rich diversity, short reaction time and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] In a Schlenk reaction tube, add 5.8 mg (20% mmol) Cu 2 O, 11.2 mg (40% mmol) 2,2'-bipyridyl, Togni reagent (chemical name: 1-(trifluoromethyl)-1,2-phenyliodyl-3(1H)-one, CAS: 887144 -94-7, 126mg, 0.4mmol), evacuate, under argon protection, add N-cyano-N-(3-methylbutyl-3-en-1-yl)benzamide (42mg, 0.2mmol ) of 2ml CH 3The CN solution was stirred and reacted at an external temperature of 80° C. for 10 h. After the reaction was completed, it was transferred to a 25 ml single-necked bottle, and the solid was loaded as a sample, and separated by column chromatography using petroleum ether and ethyl acetate as eluents. 46 mg of the product was obtained with a yield of 82%.
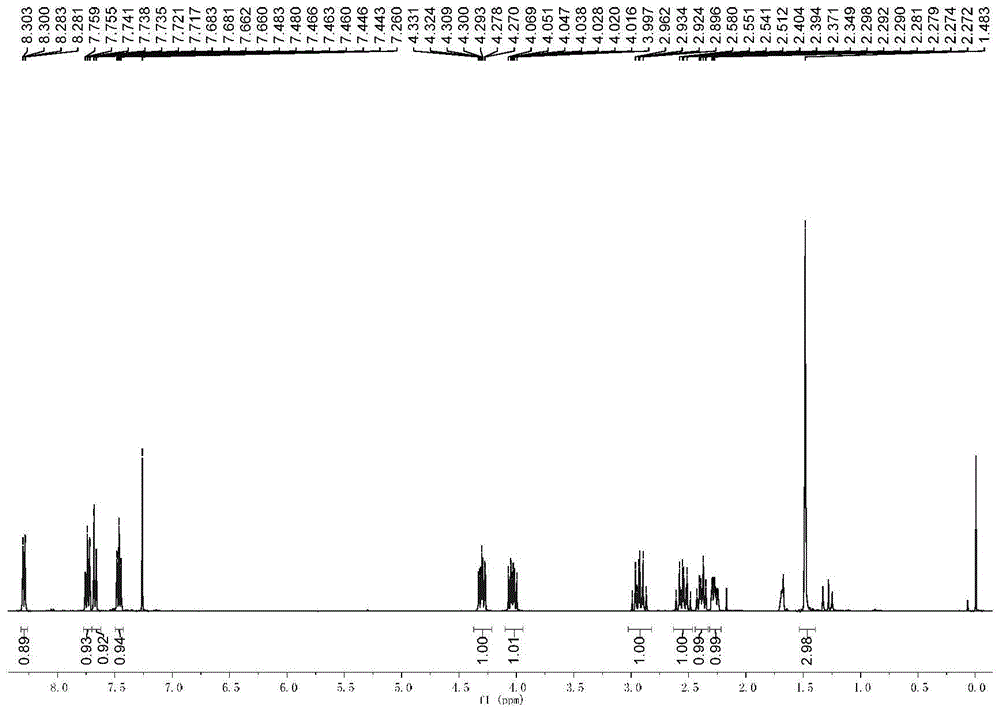
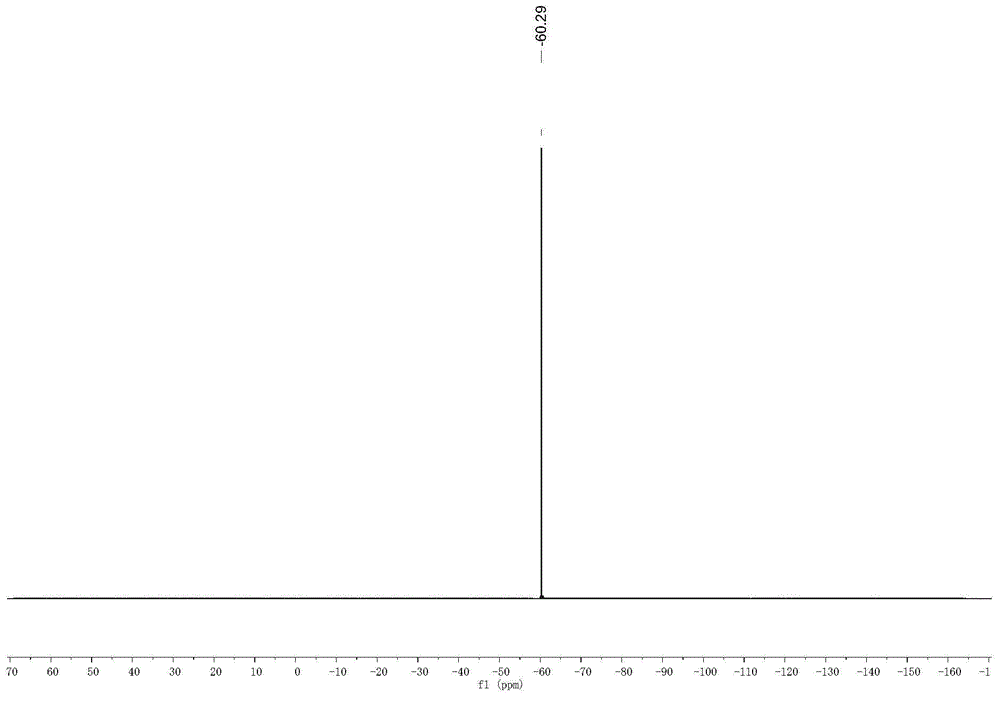
[0051] The physical properties and spectrogram data of the product are as follows: Pale yellow solid; m.p.91-92°C; 1 H NMR (CDCl 3 ,400MHz), δ:8.29(dd,J 1 =8.0Hz,J 2 =1.2Hz,1H),7.76-7.72(m,1H),7.67(dd,J 1 =8.4Hz,J 2 =0.8Hz,1H),7.46(dq,J 1 =7.4Hz,J 2 =1.2Hz,1H),4.30(dq,J 1 =11.2Hz,J 2...
Embodiment 2
[0054]
[0055] In a Schlenk reaction tube, add 5.8 mg (20% mmol) Cu 2 O, 11.2mg (40%mmol) 2,2'-bipyridyl, Togni reagent (126mg, 0.2mmol), vacuum, argon protection, add N-cyano-1-methyl-N-(3-methylbut-3 -en-1-yl)-1H-indole-2-carboxamide (57mg, 0.2mmol) in 2ml CH 3 The CN solution was stirred and reacted at an external temperature of 80° C. for 10 h. After the reaction was completed, it was transferred to a 25 ml single-necked bottle, and the solid was loaded as a sample, and separated by column chromatography using petroleum ether and ethyl acetate as eluents. 51 mg of the product was obtained with a yield of 77%.
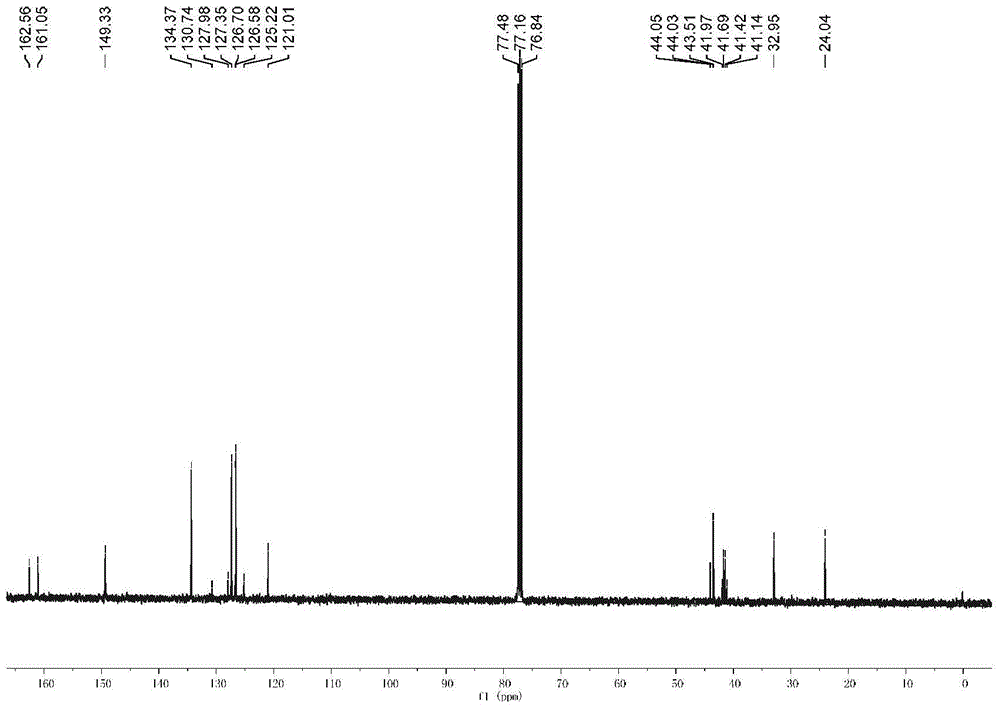
[0056] The physical properties and spectrogram data of the product are as follows: Pale yellow solid; m.p.196-197°C; 1 H NMR (CDCl 3 ,400MHz),δ:8.24(d,J=6.0Hz,1H),7.43-7.37(m,2H),7.33(m,1H),4.38(s,1H),4.12(d,J=7.2Hz, 1H),3.85(s,3H),2.96-2.85(m,1H),2.63-2.51(m,1H),2.44(s,1H),2.31-2.29(m,1H),1.49(s,3H) ; 13 C NMR (CDCl 3 ,100MHz), δ:164.92,154.53,137.48,126...
Embodiment 3~10
[0059] The preparation methods of Examples 3-10 are the same as that of Example 1, except that the two raw materials are replaced with other similar structures, and the structures of the obtained products are listed in Table 1.
[0060] The product structure of embodiment 3~10
[0061]
[0062]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com