Medicine A317491 nanometer particle and application and preparation thereof
A nanoparticle and drug technology, applied in the field of pharmaceutical preparations, can solve problems such as toxic side effects, short pain relief time, and inability to continuously relieve pain, and achieves avoiding side effects, good encapsulation and film formation, non-toxic encapsulation and formation. film effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Preparation of diclofenac-modified dextran nanoparticles loaded with drug A317491
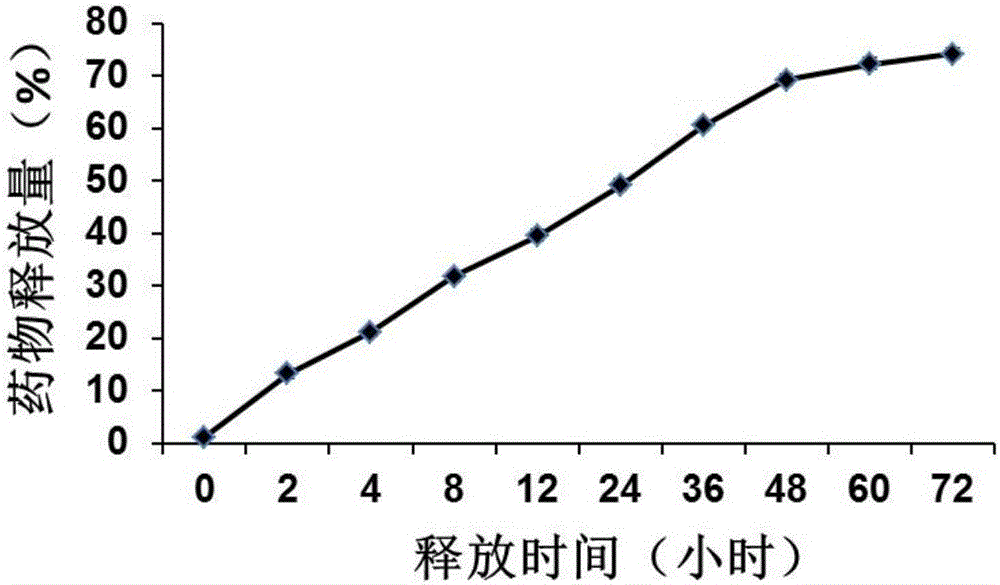
[0085] Under stirring conditions, 14.4 mg of dicyclohexylcarbodiimide (DCC) (0.07 mmol) and 20.7 mg of diclofenac (0.07 mmol) were added to 10 mL of dimethyl sulfoxide solvent, and the reaction was stirred at room temperature for 3 h; then 20mg of dextran (0.123mmol glucose unit) was added to the above reaction solution, and reacted at room temperature for 48h; the reaction solution was dialyzed for 24 hours, and freeze-dried to obtain diclofenac modified dextran; then, under stirring conditions, 20mg of the drug A317491 and 100 mg of diclofenac-modified dextran were dissolved in 20 mL of organic solvent dimethyl sulfoxide, and then added dropwise to 40 mL of hot water at 50°C in volume, and then the organic solvent was removed and freeze-dried to obtain the drug-loaded A317491-loaded diclofenac Modified dextran nanoparticles. It is determined that the drug loading amount of ...
Embodiment 2
[0086] Example 2: Preparation of diclofenac-modified dextran nanoparticles loaded with drug A317491
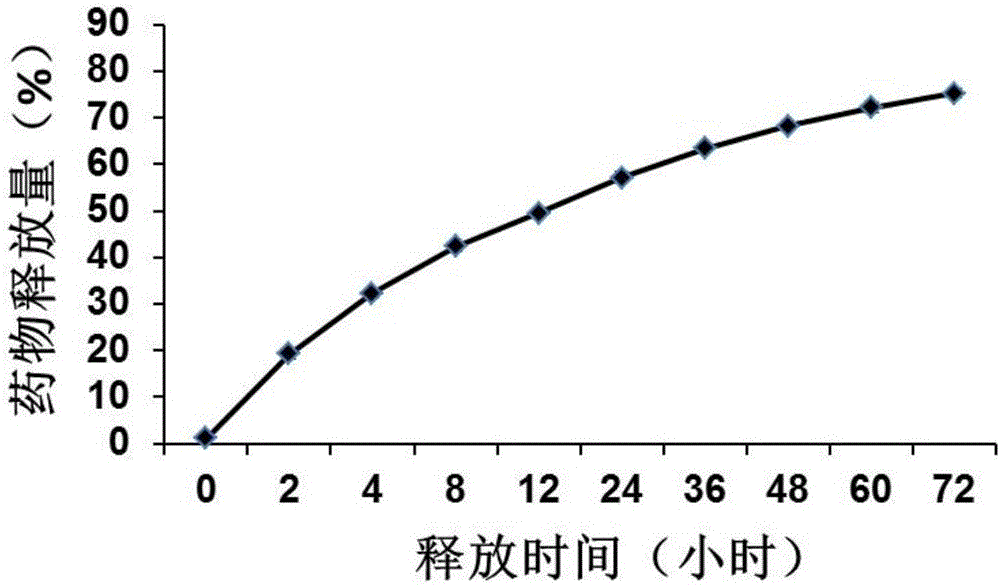
[0087]Under stirring conditions, 20.6mg of dicyclohexylcarbodiimide (DCC) (0.1mmol) and 29.6mg of diclofenac (0.1mmol) were added to 10mL of dimethyl sulfoxide solvent, and the reaction was stirred at room temperature for 3h; then 100mg dextran (0.617mmol glucose unit) was added to the above reaction solution, and reacted at room temperature for 48h; the reaction solution was dialyzed for 24 hours, and freeze-dried to obtain diclofenac modified dextran; then, under stirring conditions, 20mg of the drug A317491 and 100 mg of diclofenac-modified dextran were dissolved in 20 mL of organic solvent dimethyl sulfoxide, and then added dropwise to 40 mL of hot water at 50° C., after which the organic solvent was removed and freeze-dried to obtain the drug-loaded diclofenac A317491 Modified dextran nanoparticles. It is determined that the drug loading amount of 100 mg of nanoparticles...
Embodiment 3
[0088] Example 3: Preparation of diclofenac-modified dextran nanoparticles loaded with drug A317491
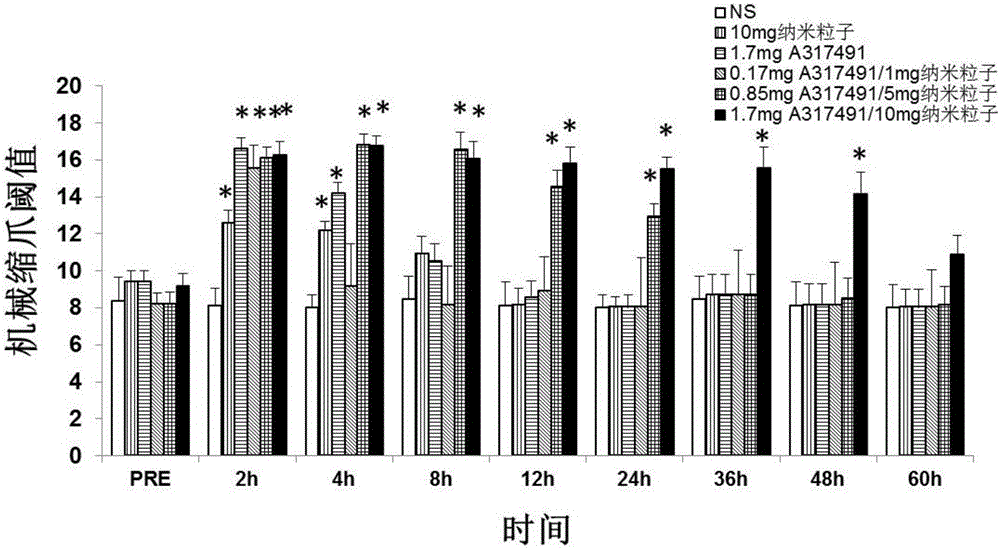
[0089] Under stirring conditions, 26.8mg of dicyclohexylcarbodiimide (DCC) (0.13mmol) and 38.5mg of diclofenac (0.13mmol) were added to 10mL of dimethyl sulfoxide solvent, and the reaction was stirred at room temperature for 3h; then 200mg of dextran (1.234mmol glucose unit) was added to the above reaction solution, and reacted at room temperature for 48h; the reaction solution was dialyzed for 24 hours, and freeze-dried to obtain diclofenac modified dextran; then, under stirring conditions, 20mg of the drug A317491 and 100 mg of diclofenac-modified dextran were dissolved in 20 mL of organic solvent dimethyl sulfoxide, and then added dropwise to 40 mL of hot water at 50° C., after which the organic solvent was removed and freeze-dried to obtain the drug-loaded diclofenac A317491 Modified dextran nanoparticles. It is determined that the drug loading amount of 100 mg of nanopar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com