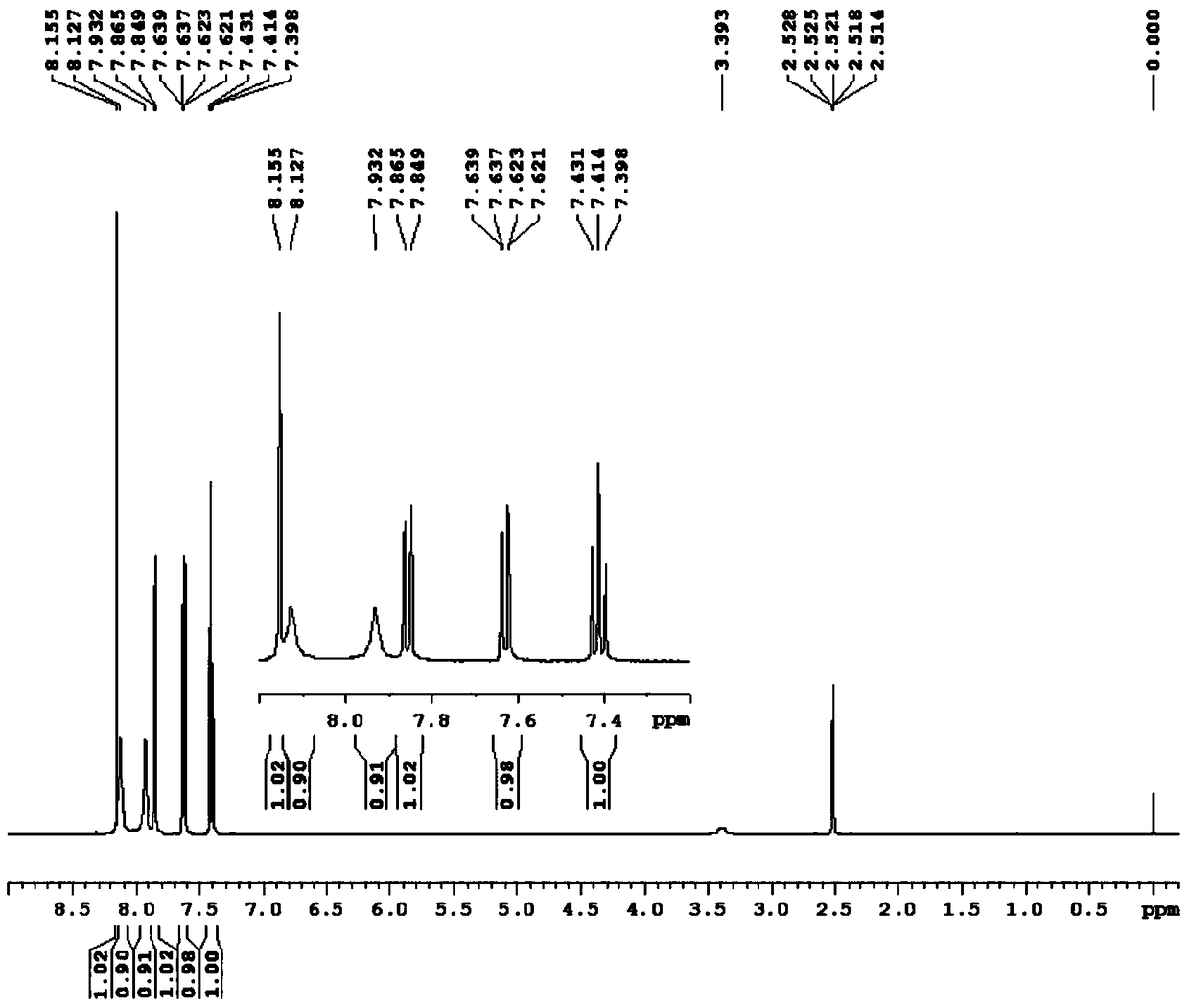
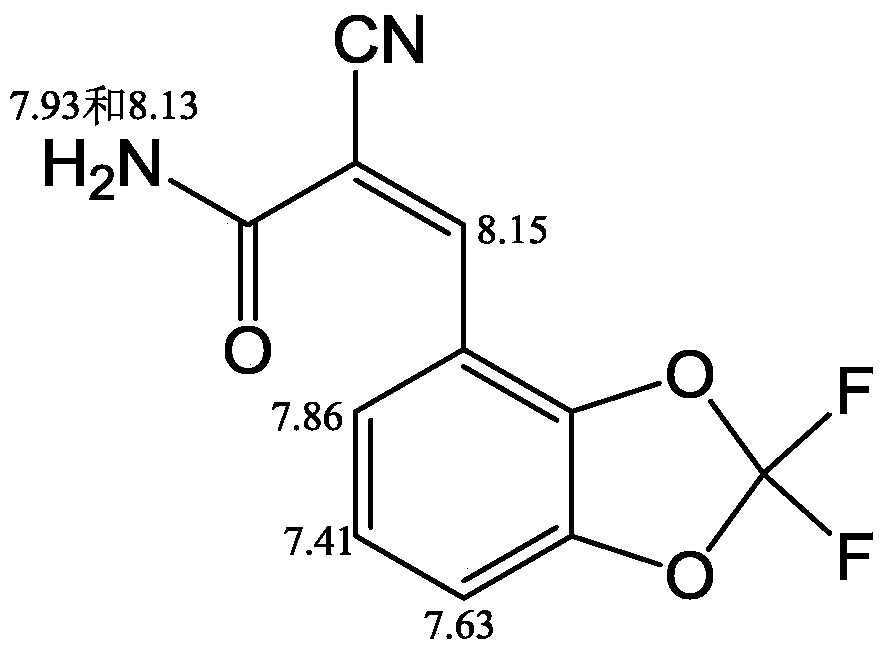
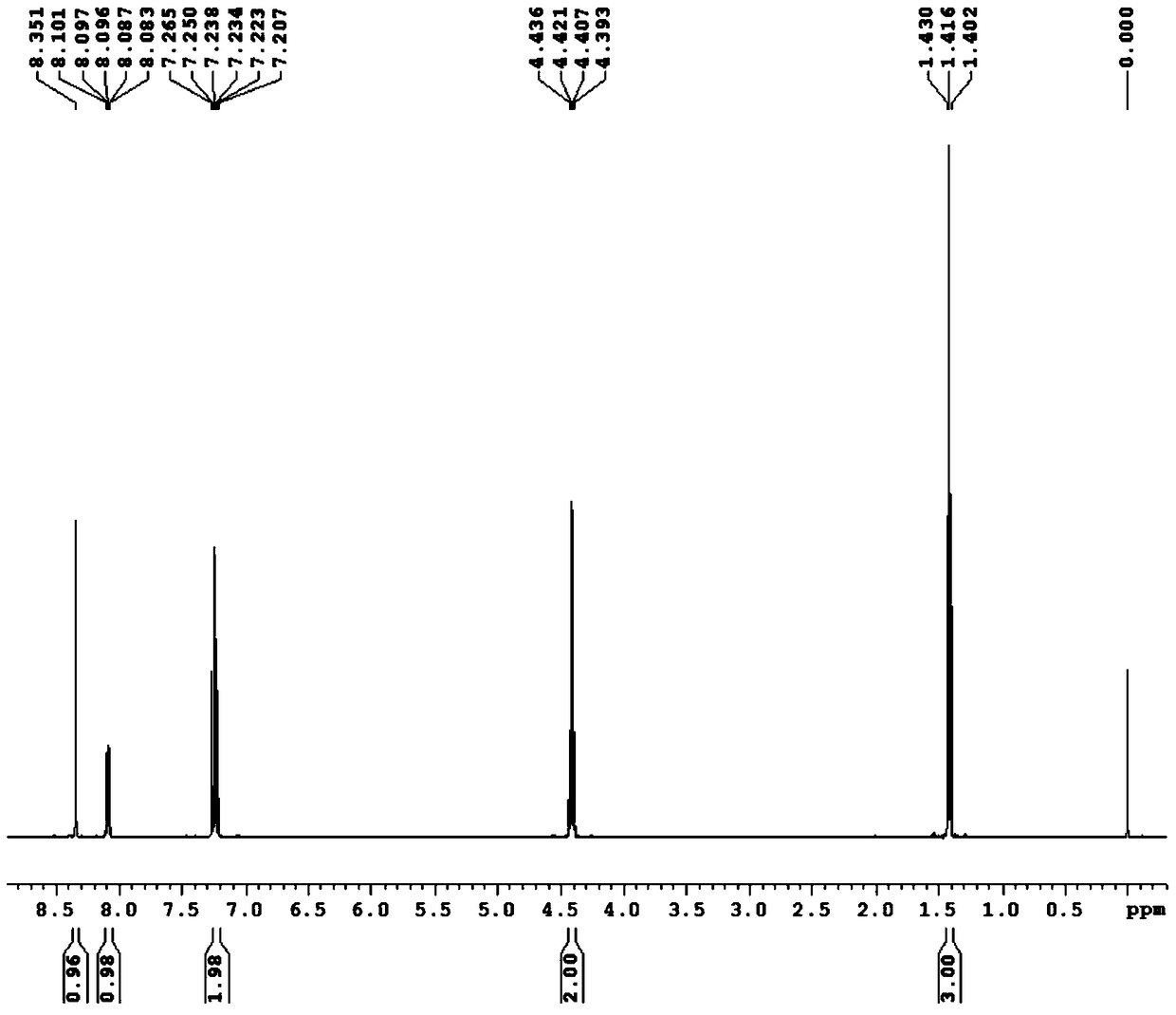
2-cyano-3-(2,2-difluoro-1,3-benzodiox-4-yl)acrylic acid compound and its preparation method
A technology of benzodioxine and compounds, which is applied in the field of organic synthesis, can solve problems such as low yield and/or purity, difficulty in solvent recovery and application, and inability to obtain intermediate products independently, so as to reduce production costs and solvent recovery loss effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-14 and comparative example 1-3
[0044] Add 2,2-difluoro-1,3-benzodiox-4-aldehyde shown in formula (II) and cyanoacetic acid derivative shown in formula (III) in the four-neck flask with mechanical stirring, then Add solvent and stir to mix well. The temperature of the mixture was lowered to the reaction temperature and kept constant, then the catalyst was added dropwise with stirring, and the stirring was continued for a certain reaction time. After the reaction is completed, filter, recover the obtained filtrate and apply it mechanically, wash the obtained filter cake and dry the washed solid at 70° C. for 12 hours to obtain the final product.
[0045] Embodiment 1-14 and the used material of the reaction of comparative example 1,3 and consumption are shown in Table 1, and reaction condition is shown in Table 2; The material of comparative example 2 and reaction condition are according to Li Chao etc. Synthesis, Modern Pesticides, 2009, 8(3), 19-21, 24).
[0046] Table 1
[0047]
[0048] Table 2
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com