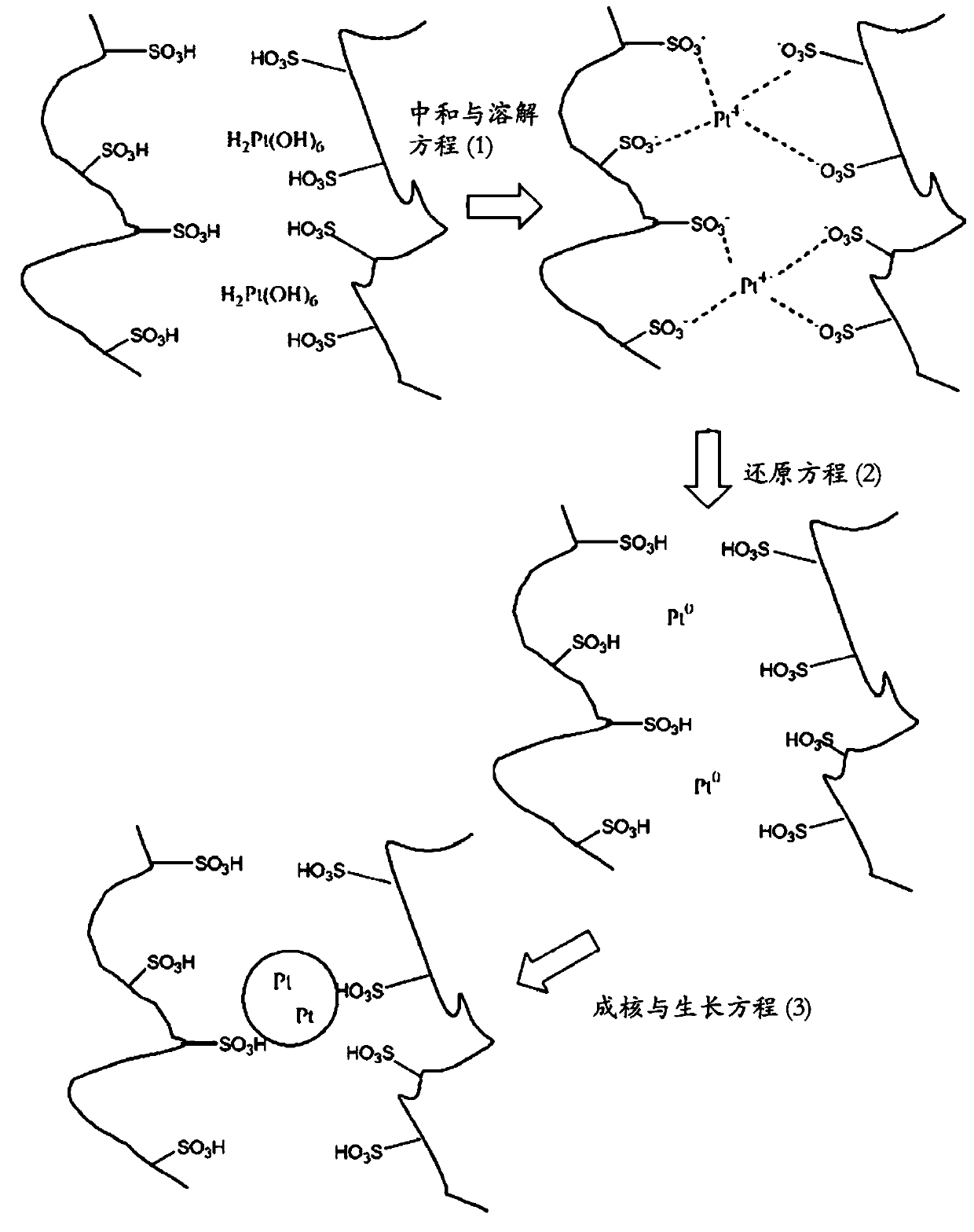
Colloidal dispersions comprising precious metal particles and acidic ionomer components and methods of their manufacture and use
A colloidal dispersion, precious metal technology, used in the field of electrodes and composite catalyst materials, the manufacture of ionomer layers, catalyst layers, and the composition of acidic ionomer compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0200] Preparation of composition (pre-product) containing Pt precursor compound and acidic ionomer
[0201] Dilute a water-based ionomer dispersion with deionized water D83-24B (from Solvay Specialty Polymers, Italy; 24% by weight ionomer; equivalent weight EW=830g / equivalent) in order to obtain an ionomer dispersion with the following composition:
[0202] 20% by weight solid ionomer
[0203] 80% by weight D.I. water
[0204] Charge 100 g of this ionomer dispersion into a 300 ml vessel. Subsequently, 375 g of grinding media (small Zr oxide beads) was added to the dispersion. Next, 1.06g platinum hexahydroxide (IV) acid H 2 Pt(OH) 6 (65% by weight Pt, Umicore AG & CoKG, Hanau / Germany) was added to the vessel and ground for 2 hours until the particle size of the Pt precursor compound reached the micron range. A yellow fluid composition was obtained, containing 0.68% by weight of Pt, 19.8% by weight of ionomer, and a Pt:ionomer ratio of 1:29.
example 2
[0206] Preparation of colloidal Pt / ionomer dispersion
[0207] A water-based ionomer dispersion D83-24B (from Solvay Specialty Polymers, Italy; 24% by weight ionomer; equivalent weight EW=830 g / equivalent) was concentrated by evaporation to obtain a 28.1% by weight ionomer dispersion in water. Add 1-propanol to this dispersion in order to obtain an ionomer dispersion with the following composition by mass:
[0208]
[0209] Charge 100 g of this ionomer dispersion into a 300 ml vessel. Subsequently, 375 g of grinding media (small Zr oxide beads) was added to the dispersion. Next, 1.06g platinum hexahydroxide (IV) acid H 2 Pt(OH) 6 (65% by weight Pt, Umicore Co., Ltd., Hanau / Germany) was added to the vessel and ground in a bead mill for 2 hours. The precursor compound is very well dispersed in the liquid ionomer, and the particles are in the micron range.
[0210] The obtained dispersion was stirred for 24 hours and reduced to produce a colloidal dispersion containing ionomer and ...
example 3
[0213] Preparation of colloidal Pt / ionomer dispersion
[0214] A water-based ionomer dispersion D83-24B (from Solvay Specialty Polymers, Italy; 24% by weight ionomer; equivalent weight EW=830 g / equivalent) was concentrated by evaporation to obtain a 28.1% by weight ionomer dispersion in water. Ethanol is added to this dispersion in order to obtain an ionomer dispersion having the following composition by mass:
[0215]
[0216] Charge 100 g of this ionomer dispersion into a 300 ml vessel. Subsequently, 375 g of grinding media (small Zr oxide beads) was added to the dispersion.
[0217] Next, 1.06g platinum hexahydroxide (IV) acid H 2 Pt(OH) 6 (65% by weight Pt, Umicore Co., Ltd., Hanau / Germany) was added to the vessel and ground in a bead mill for 2 hours. The precursor compound is very well dispersed in the liquid ionomer, and the particles are in the micron range.
[0218] The obtained dispersion was stirred for 24 hours and reduced to produce a colloidal dispersion containing i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| water solubility | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com