A kind of traditional Chinese medicine composition and its preparation method and application
A technology for a composition and a traditional Chinese medicine, applied in the field of traditional Chinese medicine composition and its preparation, can solve the problems of increasing production cost and process complexity, risks caused by consumers' health and safety, etc., and achieves the effect of reducing the content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of Chinese medicine composition of the present invention
[0040] 1. Preparation of notoginsengdiol saponins
[0041] Clean the notoginseng (head) and control the temperature at 55-60°C, dry it until there is no moisture to the touch, and then pulverize it into powder, soak it in 60% ethanol for ≥24 hours, then put it into a tube and start percolation, and control the flow rate at 5-8ml / Min kg of medicinal materials, collect the notoginseng percolation liquid, concentrate the notoginseng percolation liquid until the specific gravity is 1.08-1.12, and collect the ointment, the concentration temperature is ≤70°C, then add water and stir to form a notoginseng aqueous solution, and use macroporous adsorption resin chromatography;
[0042] The flow rate of the Panax notoginseng aqueous solution on the column is ≤600L / h. After loading the column, first wash with water at 800L / h for half an hour, then with water at 1200L / h for half an hour, and f...
Embodiment 2
[0053] Embodiment 2: the preparation of liver protection tablet
[0054] Mix 25 parts of kudzu root extract, 10 parts of salvia miltiorrhiza extract, 150 parts of notoginseng glycol saponins, 60 parts of microcrystalline cellulose, 120 parts of pregelatinized starch, and 120 parts of dextrin with a three-dimensional mixer for 10 minutes; then use 16 parts 14% starch slurry was prepared by starch boiling method; the starch slurry was mixed with the above mixture, and wet granulation was carried out with a swinging vibrating sieve; the granules were dried in a hot air circulation oven at 60°C for 3 hours, and then taken out; The water content of the granules is checked by an instrument (less than 10%); 2.5 parts by weight of magnesium stearate are added for total mixing and then compressed into tablets; and then film-coated to obtain the final product.
Embodiment 3
[0055] Embodiment 3: Security testing
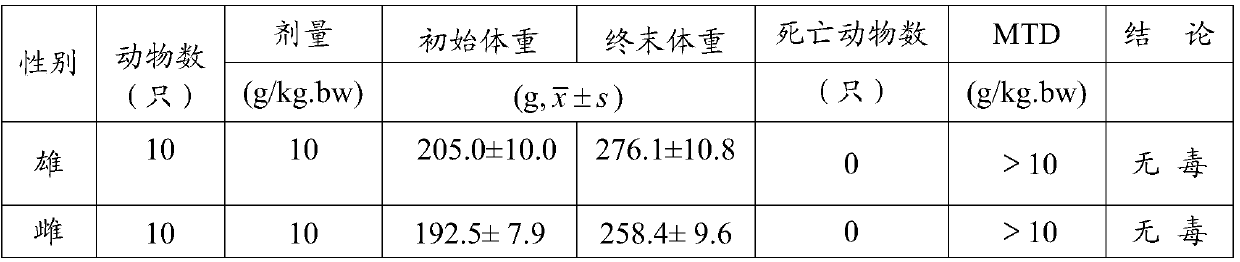
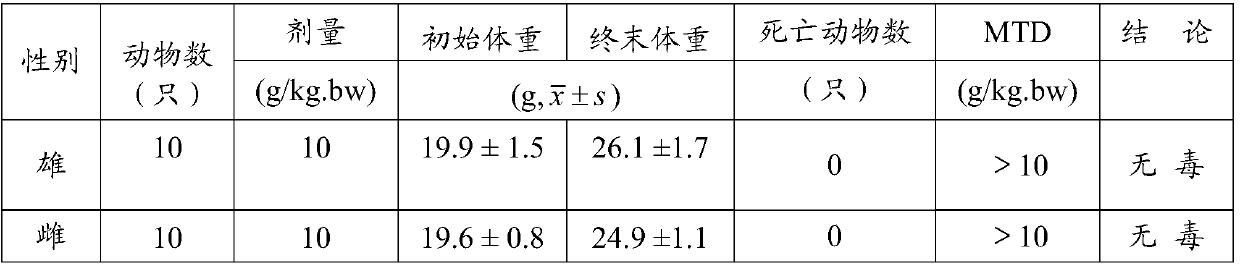
[0056] 1. Experimental animals: 20 SPF grade SD rats, half male and half female, weighing 180g-220g; 20 SPF Kunming mice, half male and half female, weighing 18g-22g, all provided by Chengdu Dashuo Biotechnology Co., Ltd. Certificate number: SCXK (Sichuan) 2008-24. Quarantine for one week before the test. During the whole test, the animals were free to eat and drink, the room temperature was 21°C-24°C, and the relative humidity was 50%-65%.
[0057] 2. Test method
[0058] Acute oral toxicity test in rats: taking the traditional Chinese medicine composition 1 of the present invention as the detection object, the maximum tolerated dose method was used to conduct the test, and a dose group of 10 g / kg was set up in the test. There were 10 male and female SD rats in each group. Sample preparation: Accurately weigh 50g of the sample, dissolve it in distilled water and make up to 200ml, orally orally gavage the animal twice with an interval...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com