Betrixaban salt, preparation method and application thereof
A technology of betrixaban and malic acid, applied in the field of organic chemistry, can solve problems such as difficult control of polycrystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
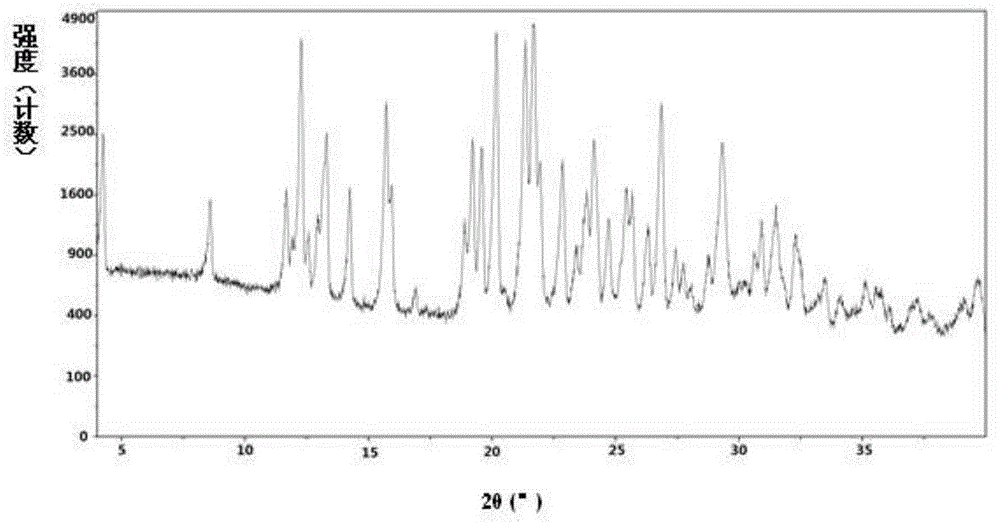
[0260] Example 1: Preparation of semi-L-betrixaban malate and its crystal form A
[0261] Dissolve 6.0g (13.3mmol) of betrixaban and 1.1g (8.0mmol) of L-malic acid in a mixed solvent of 70mL of tetrahydrofuran / 7mL of water at 55-60°C, add 60mL of acetone while stirring, and cool to room temperature. Crystallization. Precipitate a solid, filter it, and dry the obtained solid under reduced pressure at 40-45°C to obtain half-L-betrixaban malate.
[0262] 1 HNMR(400MHz,MeOD)δ:2.355-2.419(dd,0.5H),2.735-2.781(dd,0.5H),3.226(s,6H),3.907(s,3H),4.302-4.326(dd,0.5H) ),7.195-7.224(dd,1H),7.448-7.455(d,1H),7.744-7.764(d,2H),7.821-7.849(dd,1H),8.145-8.165(d,2H),8.196-8.219 (d,1H),8.238-8.261(d,1H),8.323-8.329(d,1H).
[0263] the above 1 In the H-NMR results, δ: 3.907(s,3H) is attributed to the methyl CH in the betrixaban molecule 3 , 4.302-4.326 (dd, 0.5H) is attributed to the methine CH in the L-malic acid molecule. It can be judged that the molar composition ratio of betrixaban an...
Embodiment 2
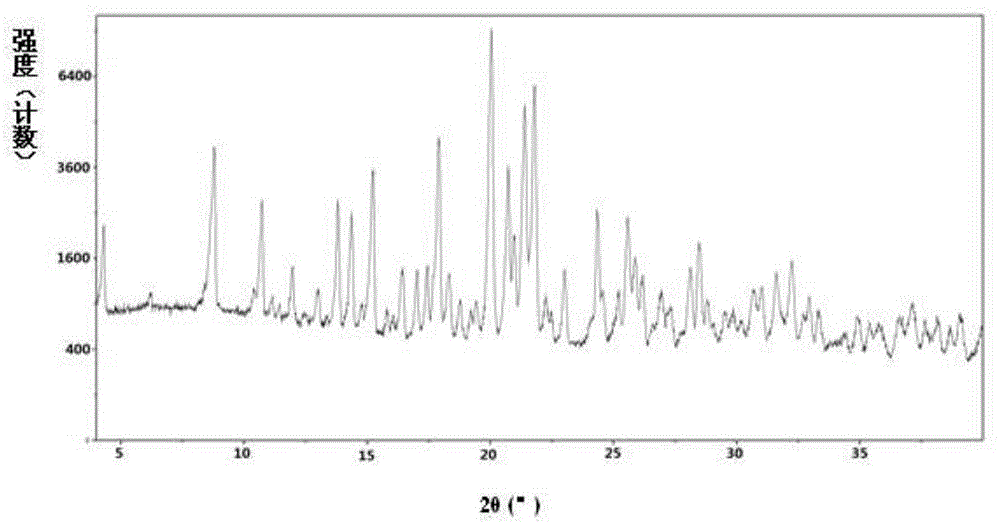
[0268] Example 2: Preparation of L-betrixaban malate and its crystal form A
[0269] Dissolve 6.0g (13.3mmol) of betrixaban (13.3mmol) and 2.1g (16.0mmol) of L-malic acid in 48mL of tetrahydrofuran / 12mL of water mixed solvent at 55-60°C, add 80mL of acetone dropwise under stirring, and cool to room temperature. Stand still and crystallize. Precipitate a solid, filter it, and dry the obtained solid under reduced pressure at 45-50°C to obtain L-betrixaban malate.
[0270] 1 HNMR(400MHz,MeOD)δ:2.491-2.549(dd,1H),2.779-2.832(dd,1H),3.122(s,3H),3.350(s,3H),3.925(s,3H),4.267-4.300 (dd,1H),7.220-7.250(dd,1H),7.474-7.481(d,1H),7.755-7.776(d,2H),7.841-7.869(dd,1H),8.164-8.185(d,2H) ,8.207-8.230(d,1H),8.275-8.298(d,1H),8349-8.357(d,1H).
[0271] the above 1 In the H-NMR results, δ: 3.925(s,3H) is attributed to the methyl CH in the betrixaban molecule 3 , δ: 4.267-4.300 (dd, 1H) is attributed to the methine CH in the L-malic acid molecule. It can be judged that the molar compositio...
Embodiment 3
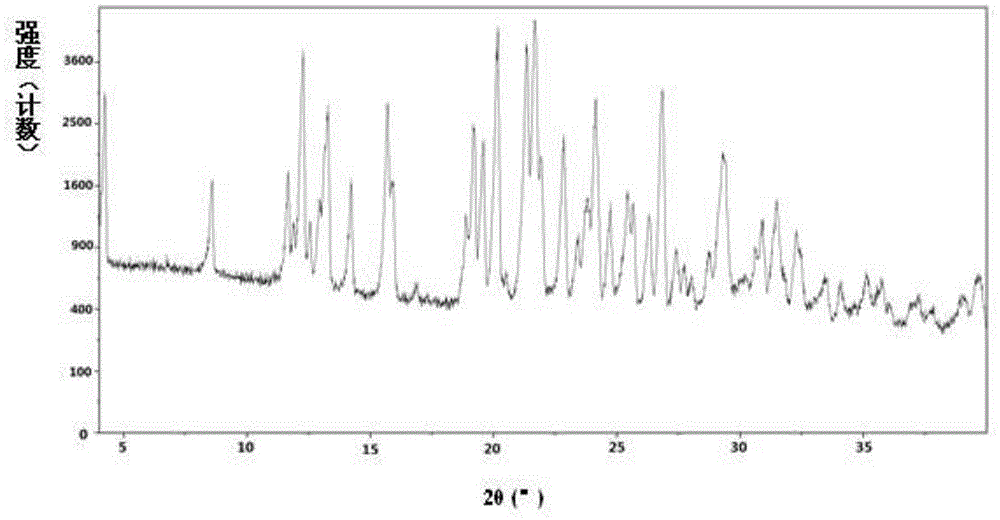
[0276] Example 3: Preparation of hemi-D-betrixaban malate and its crystal form A
[0277] Dissolve 6.0g (13.3mmol) of betrixaban (13.3mmol) and 1.1g (8.0mmol) of D-malic acid in a mixed solvent of 70mL of tetrahydrofuran / 7mL of water at 55-60°C, add 60mL of acetone dropwise under stirring, and cool to room temperature. Stand still and crystallize for 12h. Precipitate a solid, filter it, and dry the obtained solid under reduced pressure at 50-55°C to obtain betrixaban hemi-D-malate.
[0278] 1 HNMR(400MHz,MeOD)δ:2.492-2.540(dd,0.5H),2.763-2.771(dd,0.5H),3.321(s,3H),3.148(s,3H),3.927(s,3H),4.277 -4.301(dd,0.5H),7.223-7.253(dd,1H),7.474-7.481(d,1H),7.755-7.775(d,2H),7.843-7.871(dd,1H),8.163-8.183(d ,2H),8.207-8.2229(d,1H),8.268-8.290(d,1H),8.352-8.358(d,1H).
[0279] the above 1 In the H-NMR results, δ: 3.927(s,3H) is attributed to the methyl CH in the betrixaban molecule 3 , δ: 4.277-4.301 (dd, 0.5H) is attributed to the methine CH in the D-malic acid molecule. It can be j...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com