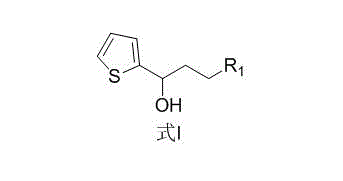
New preparation method of duloxetine intermediate
A technology for duloxetine and intermediates, which is applied in the field of preparation of duloxetine intermediates, and can solve the problems of high yield and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of 3-chloro-1-(2-thiophene)-1-propanol
[0024]
[0025] Add 50 g (0.286 mol) of 3-chloro-1-(2-thiophene)-1-propanone and 300 mL of isopropanol to the reaction flask, stir well, add 175 g (0.858 mol) of aluminum isopropoxide and 3.8 g of aluminum trichloride g (0.0286mol), the temperature was raised to 75°C, and the reaction was stirred for 6 hours. After the completion of the reaction, cool down to room temperature, add 600 mL of water and adjust the pH to 5.0 with hydrochloric acid, extract with ethyl acetate (300 mL×3), dry over sodium sulfate, and evaporate to dryness under reduced pressure to give 3-chloro-1-(2-thiophene)-1 -Propanol oil 43.8g, yield 86.7%, purity 97.2%. 1 HNMR (CDCl 3 ): 7.26(m,1H,Ar-H), 6.99(m,2H,Ar-H), 5.21(m,1H,CH), 3.73-3.60(m,2H,CH-CH 2 ), 2.24(m,2H,Cl-CH 2 ).
Embodiment 2
[0026] Example 2: Preparation of 3-bromo-1-(2-thiophene)-1-propanol
[0027]
[0028] Add 10g (0.046mol) of 3-bromo-1-(2-thiophene)-1-propanone and 50mL of tert-butanol into the reaction flask, stir well, add 56.4g (0.276mol) of aluminum isopropoxide, and heat up to 70°C , stirred for 5 hours. After the reaction was completed, cool down to room temperature, add 100 mL of water, adjust the pH to 7.0 with 6M sulfuric acid, extract with ethyl acetate (50 mL×3), dry over sodium sulfate, and evaporate to dryness under reduced pressure to give 3-bromo-1-(2-thiophene) -1-propanol oil 9.3g, yield 91.3%, purity 95.4%.
Embodiment 3
[0029] Example 3: 3-Bromo-1-(2-thiophene)-1-propanol refined
[0030] Take 5.0g of crude 3-bromo-1-(2-thiophene)-1-propanol (purity 95.4%), add methanol 10mL, isopropyl ether 40mL, heat to reflux to dissolve, cool to room temperature, stir for 1 hour, 0~ Stir at 5°C for 3 hours, filter with suction, and dry under normal pressure to obtain 4.5 g of solid, with a yield of 90.0% and a purity of 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com