Bithiophene pyrrolic compound containing diphenyl-4-phenylamine diphenyl diamine and preparation as well as polymer and preparation method and application
An aniline biphenyl diamine group and bisthieno technology, which is applied to the bis thienopyrrole compound containing diphenyl-4-aniline biphenyl diamine group and the preparation and polymer and preparation and application fields, can solve the problem of discoloration Single, electrochromic polymer film-forming problems, etc., to achieve the effect of good electrochromic performance and film-forming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
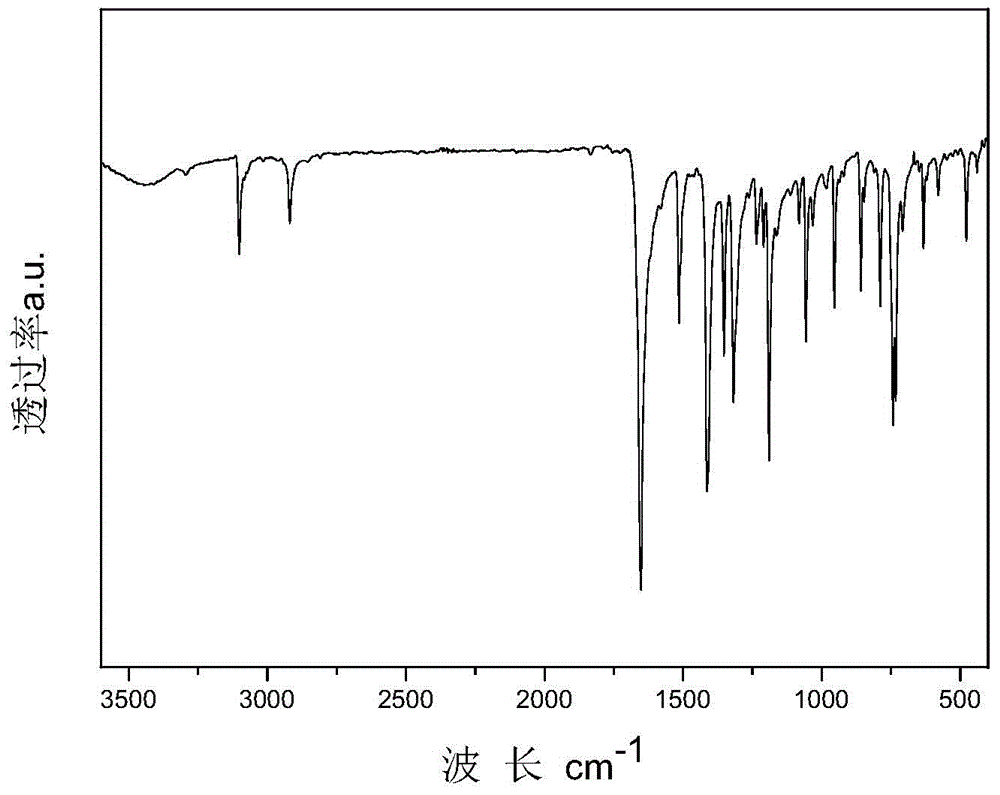
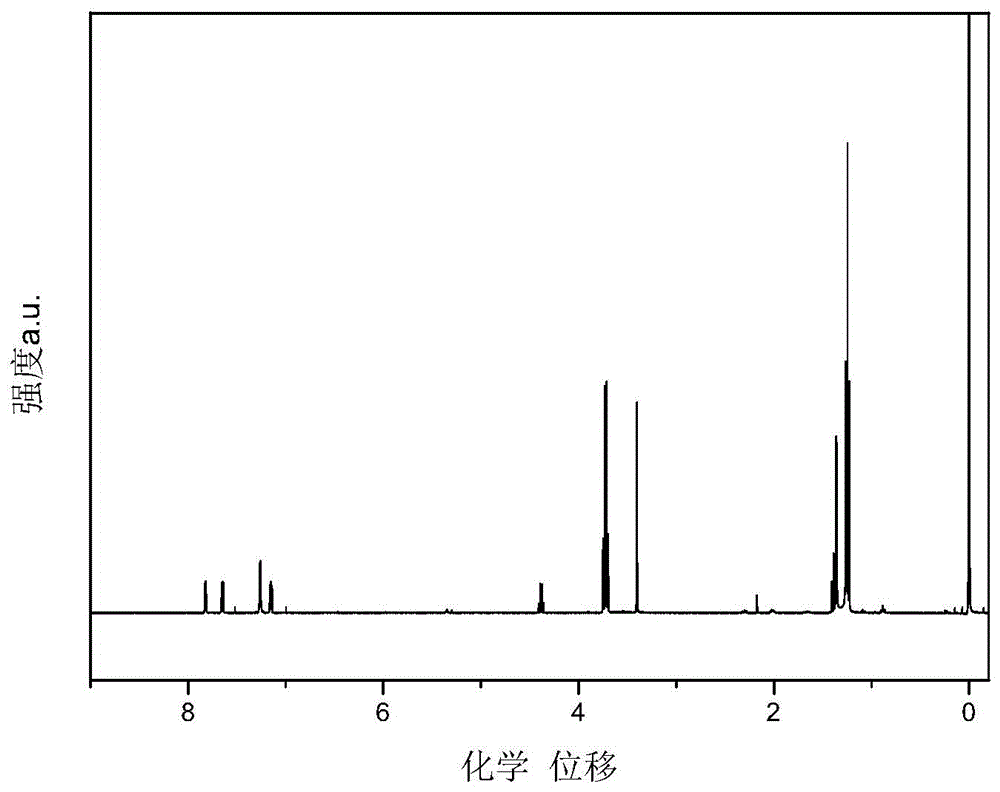
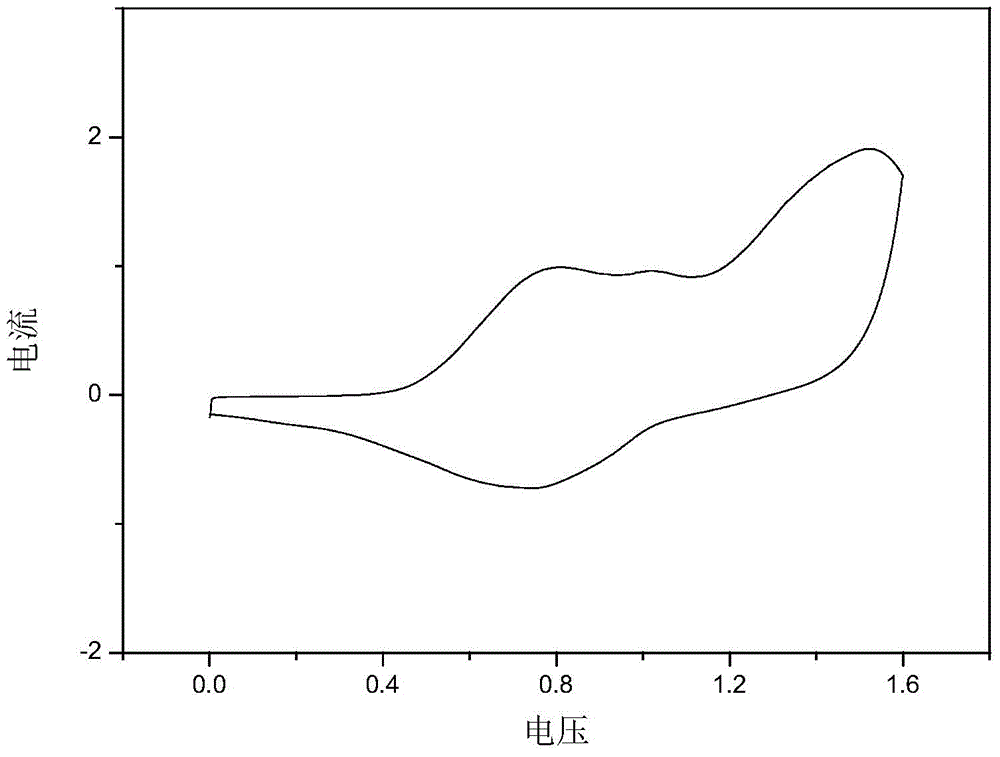
[0057] Specific embodiment one: In this embodiment, the structural formula of the bisthienopyrrole compound containing diphenyl-4-anilinebiphenylenediamine group is:
[0058]
specific Embodiment approach 2
[0059] Specific embodiment two: this embodiment is the preparation method of bisthienopyrrole compound containing diphenyl-4-aniline biphenyl diamine group is prepared according to the following method:
[0060] 1. Synthesis of 2,5-bis(2-thiophene)-1,4-butanedione monomer:
[0061] 1. Aluminum trichloride is dispersed in anhydrous dichloromethane to obtain a suspension of aluminum trichloride and anhydrous dichloromethane;
[0062] The volume ratio of the amount of aluminum trichloride described in step 1. to anhydrous dichloromethane is (0.06mol~0.1mol): 30mL;
[0063] 2. Add the mixed solution of thiophene and succinoyl chloride / dichloromethane dropwise to the suspension of aluminum trichloride and anhydrous dichloromethane obtained in step 1. respectively at a rate of 20 drops / min to 30 drops / min. In the turbid liquid, stir and react at a stirring speed of 600r / min to 700r / min for 7h to 9h, then add ice and hydrochloric acid with a mass fraction of 35% to 37%, and then sti...
specific Embodiment approach 3
[0088]Specific embodiment three: the difference between this embodiment and specific embodiment two is: the 2,5-bis(2-thiophene)-1,4-butanedione monomer described in step three and N,N'- The molar ratio of diphenyl-N,N'-bis(4-phenylamine)benzidinediamine is 0.002:0.001. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com