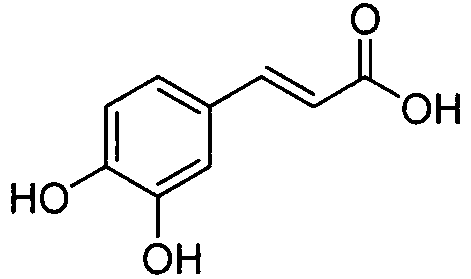
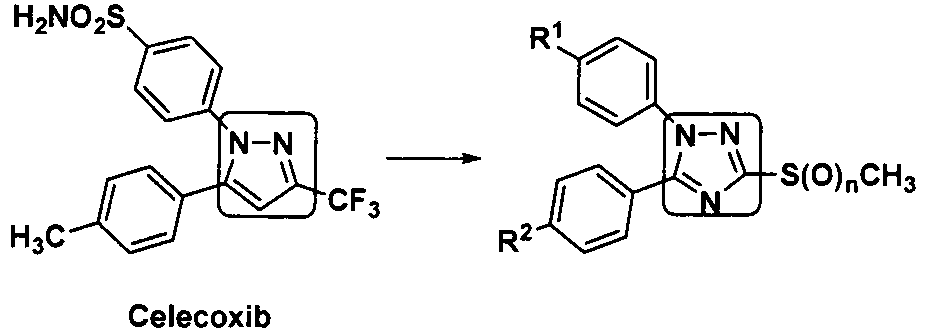
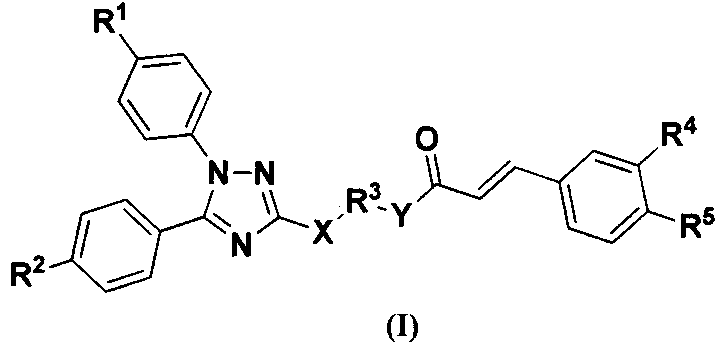
Benzene acylated 1,5-diaryl-1,2,4-triazole derivative, its preparation method and medical use
A technology of phenylacryloyl and acryloylation, which is applied in the production of bulk chemicals, drug combination, anti-tumor drugs, etc., can solve the problems that 5-LOX has no obvious inhibitory effect and no anti-tumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] Preparation of 1-(4-bromophenyl)-3-thiosemicarbazide
[0194] Suspend p-bromophenylhydrazine hydrochloride (5.0g, 0.022mol) in 20mL of absolute ethanol, add 0.5mL of concentrated hydrochloric acid, raise the temperature to 40°C, add KSCN (2.6g, 0.027mol) in batches within 40min, and complete the addition , heated up to reflux for 10h, ended the reaction, filtered while it was hot, washed the filter cake with ethanol (3 × 5mL) and discarded; after most of the solvent was evaporated from the filtrate under reduced pressure, water was added to precipitate a large amount of precipitate, left to stand, filtered with suction, The filter cake was washed with ice water to obtain 2.5 g of dark red solid, yield: 45.6%. m.p.198-200℃; ESI-MSm / z: 245.8[M-H] - .
Embodiment 2
[0196] Preparation of 4-methylsulfonylbenzoyl chloride
[0197] p-Methanesulfonylbenzoic acid (10g, 0.050mol) was suspended in 50mL of dry dichloromethane, oxalyl chloride (15mL, 0.150mol) was added, catalyzed by adding 1 drop of DMF, stirred at room temperature for 3h, the reaction was stopped, and the reaction solution was Concentrate to dryness under reduced pressure to obtain 10.1 g of white solid, yield: 92.6%, m.p.131-133°C.
Embodiment 3
[0199] Preparation of 2-(4-methylsulfonylbenzoyl)-2-(4-bromophenyl)thiosemicarbazide
[0200] Dissolve 1-(4-bromophenyl)-3-thiosemicarbazide (0.83g, 3.4mmol) in 20mL of dry acetone, drop triethylamine 5d into it, stir at room temperature, add compound 1 in batches within 30min ( 0.74g, 3.4mmol), the reaction solution gradually became turbid, after the addition, the temperature was raised to reflux for 2h, the reaction was terminated, cooled, suction filtered, the filter cake was washed with acetone (3×5mL), and dried to obtain a white solid 1.05g, the yield : 72.6%. m.p.178-180℃; ESI-MS m / z: 427.9[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com