Preparation method of permitil
A technology for preparing fluphenazine hydrochloride, which is applied in the field of preparation of fluphenazine hydrochloride, can solve problems such as increased production costs, substandard product quality, side reactions, etc. Good, the effect of reducing the difficulty of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
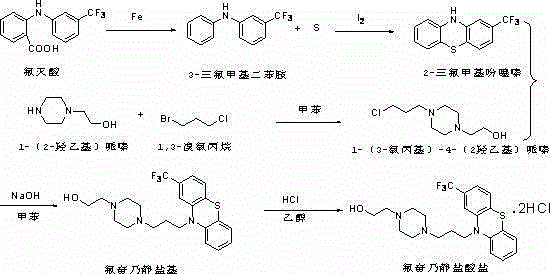
[0012] (1) Preparation of 2-trifluoromethylphenothiazine: Add 100g (0.356mol) flufenamic acid into the reaction flask, heat to 180-190°C, start stirring, and add 10g into the reaction flask after it is completely melted (0.178mol) iron powder, stirred and reacted at 180-190°C for about 2 hours. After the reaction, when the reaction solution was cooled to 100°C, pour the reaction solution into the beaker while it was still hot, and the iron powder remained at the bottom of the reaction bottle (available water rinse). The reaction solution was added to a clean reaction flask and distilled under reduced pressure, and the fraction at 134-135°C (3mmHg) was collected to obtain about 67.5 g of light yellow liquid 3-trifluoromethyldiphenylamine, with a yield of about 80%.
[0013] Add 60g (0.253mol) of 3-trifluoromethyldiphenylamine and 8g (0.253mol) of sublimed sulfur into the reaction flask, heat up to about 130°C under stirring, add 3g of iodine to the reaction flask after the sulf...
Embodiment 2
[0018] (1) Preparation of 2-trifluoromethylphenothiazine: Add 500g (1.78mol) flufenamic acid into the reaction flask, heat to 180-190°C, start stirring, and add 50g into the reaction flask after it is completely melted (0.89mol) iron powder, stirred and reacted at 180-190°C for about 2 hours. After the reaction, when the reaction solution was cooled to 100°C, pour the reaction solution into the beaker while it was still hot, and the iron powder remained at the bottom of the reaction bottle (available water rinse). The reaction solution was added to a clean reaction flask and distilled under reduced pressure, and the fraction at 134-135°C (3mmHg) was collected to obtain about 346g of light yellow liquid 3-trifluoromethyldiphenylamine, with a yield of about 82%.
[0019] Add 300g (1.265mol) of 3-trifluoromethyldiphenylamine and 40g (1.265mol) of sublimed sulfur into the reaction flask, heat up to about 130°C under stirring, add 15g of iodine to the reaction flask after the sulfu...
Embodiment 3
[0024] (1) Preparation of 2-trifluoromethylphenothiazine: Add 1000g (3.56mol) flufenamic acid into the reaction flask, heat to 180-190°C, start stirring, and add 100g into the reaction flask after it is completely melted (1.78mol) iron powder, stirred and reacted at 180-190°C for about 2 hours. After the reaction, when the reaction solution was cooled to 100°C, pour the reaction solution into the beaker while it was still hot, and the iron powder remained at the bottom of the reaction bottle (available water rinse). The reaction solution was added to a clean reaction flask and distilled under reduced pressure, and the fraction at 134-135°C (3mmHg) was collected to obtain about 1029g of light yellow liquid 3-trifluoromethyldiphenylamine, with a yield of about 82%.
[0025] Add 600g (2.53mol) of 3-trifluoromethyldiphenylamine and 80g (2.53mol) of sublimed sulfur into the reaction flask, heat up to about 130°C under stirring, add 30g of iodine to the reaction flask after the sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com