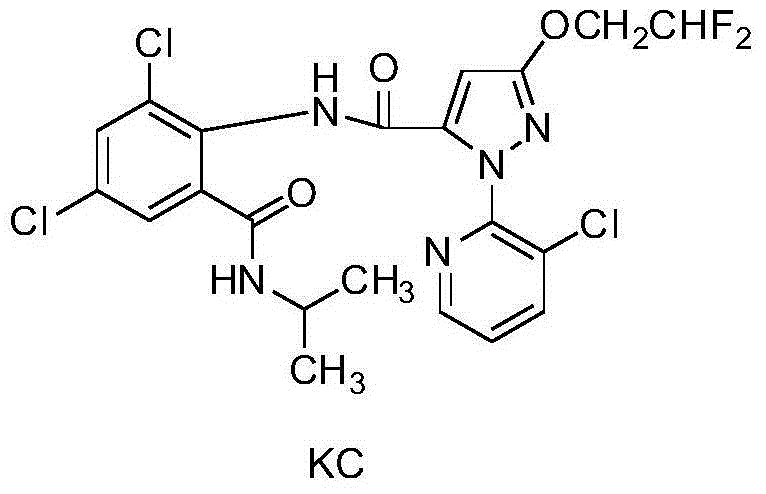
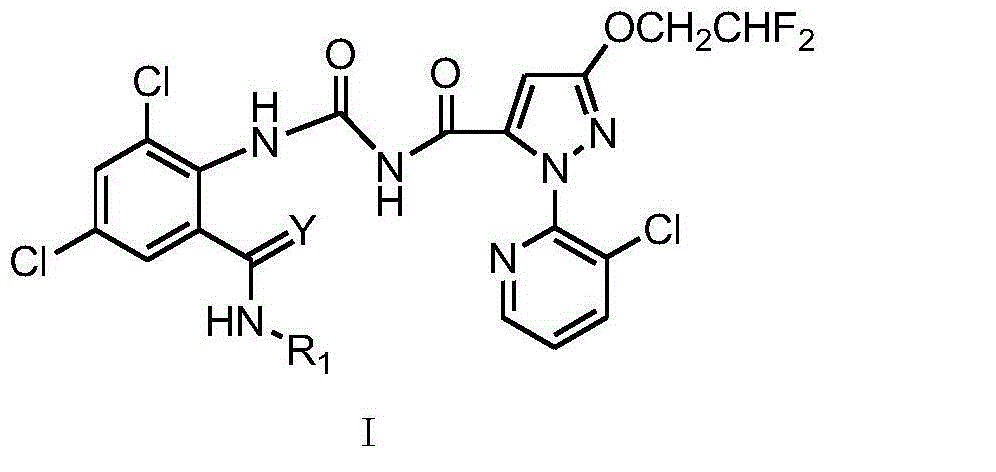
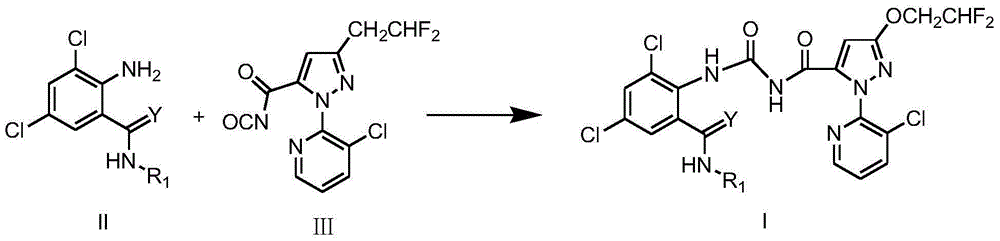
Pyrazolopyridine ureide compound and application thereof
A technique for pyridylpyrazole urea and compounds, which is applied in the field of agricultural pesticides and can solve the problems that pyridylpyrazole urea compounds have not been disclosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] The preparation of example 1, compound 1
[0024] (1) Synthesis of 2-amino-N-furanmethylbenzamide
[0025]
[0026] Add 16.3g (0.1mol) isatoic anhydride and 180mL water to a 250mL reaction flask, drop in 14.55g (0.15mol) furfurylamine (2-furylmethylamine), react at room temperature for 12h, filter with suction, filter cake in 10mL×2 Washed with tap water and dried to obtain 18.4 g of white solid with a yield of 85%.
[0027] (2) Synthesis of 2-amino-3,5-dichloro-N-furanmethylbenzamide
[0028]
[0029] Add 21.6g (0.1mol) of 2-amino-N-furylmethylbenzamide and 150g of ethyl acetate to a 250mL reaction flask, add 33.75g (0.25mol) of sulfuryl chloride dropwise at a temperature below 15°C, and control the temperature after dropping After reacting at 15°C for 4-5 hours, TLC detected that the raw materials disappeared, filtered with suction, washed the filter cake with 20 mL×2 ethyl acetate, and dried to obtain 22.37 g of white solid, with a yield of 78.5%.
[0030] (...
example 2
[0039] The preparation of example 2, compound 2
[0040] (1) Preparation of 2-amino-N-isopropylbenzamide
[0041]
[0042] Add 16.3g (0.1mol) isatoic anhydride, 100ml ethyl acetate, 1.5g glacial acetic acid to a 250mL reaction flask, add 0.15mol isopropylamine dropwise at 40-50°C, continue to react at room temperature for 2 hours, and evaporate the solvent , to obtain a white powdery solid, washed with water to remove isopropylamine hydrochloride, and dried to obtain 15.1 g of a white solid, with a yield of 85.8%.
[0043] (2) Synthesis of 2-amino-3,5-dichloro-N-isopropylbenzamide
[0044]
[0045] Add 17.8 g (0.1 mol) of 2-amino-N-isopropylbenzamide and 100 mL of ethyl acetate to a 250 mL reaction flask, and add SO 2 Cl 2 33.75g (0.25mol), after dropping, stirred for 4h, suction filtered and washed with water to obtain 20.7g of off-white solid with a yield of 83.8%.
[0046] (3) Synthesis of Compound 2
[0047]
[0048] Add 2.47g (0.01mol) 2-amino-3,5-dichloro-N...
example 3
[0049] The preparation of example 3, compound 3
[0050] (1) Synthesis of 2-amino-3,5-dichloro-N-isopropylthiobenzamide
[0051]
[0052] Add P to the 500mL reaction bottle 2 S 5 11.1g (0.05mol), 150mL of dichloromethane, stirred at room temperature, added 24.7g (0.1mol) of 2-amino-3,5-dichloro-N-isopropylbenzamide, heated to reflux for 1h until the reaction of raw materials was completed , add 150mL of water, neutralize pH 6-7 with 20% NaOH aqueous solution, evaporate dichloromethane to obtain 24.2g of yellow solid, yield 92%.
[0053] (2) Synthesis of compound 3
[0054]
[0055] Add 2.63g (0.01mol) 2-amino-3,5-dichloro-N-isopropylthiobenzamide and 10mL dichloroethane to a 100mL reaction flask, add 3.61g (0.011mol) 1-( 3-Chloropyridin-2-yl)-3-difluoroethoxy-1-H-pyrazole-5-formyl isocyanate diluted in 6mL dichloroethane, reacted at room temperature for 4h to 2-amino-3,5 Dichloro-N-furanmethylbenzamide disappeared, added 20g of water, distilled out dichloromethane, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com