Release detection method of aniracetam sustained release tablet
A detection method and technology of Aniracetam are applied in the field of drug release detection of Aniracetam sustained-release tablets, and achieve the effects of being convenient for large-scale industrial production, large application value and simple operation of the method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Drug release detection method
[0031] A drug release detection method for Aniracetam sustained-release tablets
[0032] (1) Selection of maximum absorption wavelength
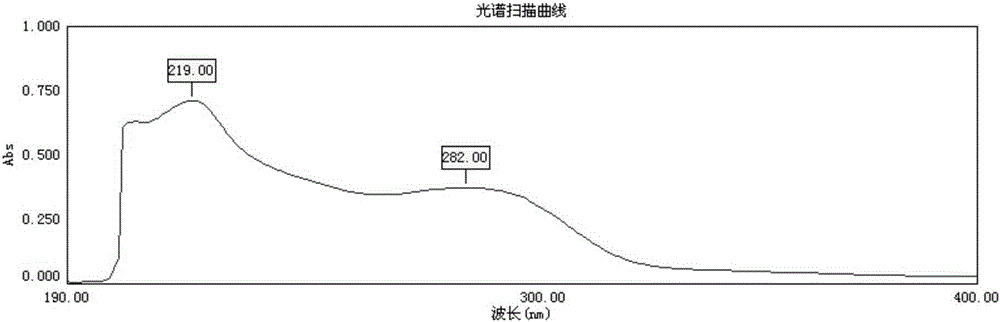
[0033] Accurately weigh 5.02mg of Aniracetam reference substance, put it in a 50ml volumetric flask, add a small amount of ethanol to dissolve it, dilute it to the mark with hydrochloric acid solution (9→1000), draw 1.0mL into a 100mL volumetric flask, and dissolve it with hydrochloric acid solution (9→1000). 1000) diluted to scale, according to "Chinese Pharmacopoeia" 2010 edition appendix IVA ultraviolet spectrophotometry, carry out ultraviolet scanning, the result has maximum absorption at 282nm, so the selection detection wavelength is 282nm. See figure 1 .
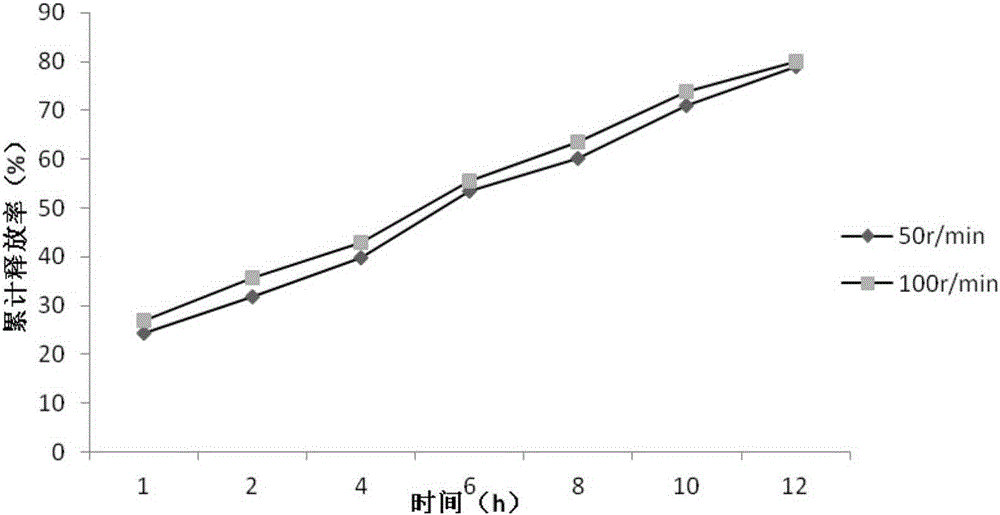
[0034] (2) The influence of rotational speed on the release rate of the main drug
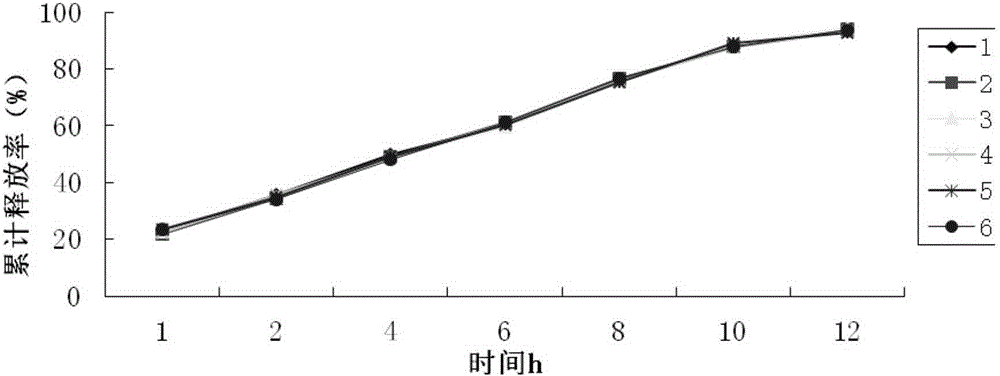
[0035] Get this product, according to the release assay method, adopt the release assay method (Chinese Pharmacopoeia 2010 version two appendix XD second me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com