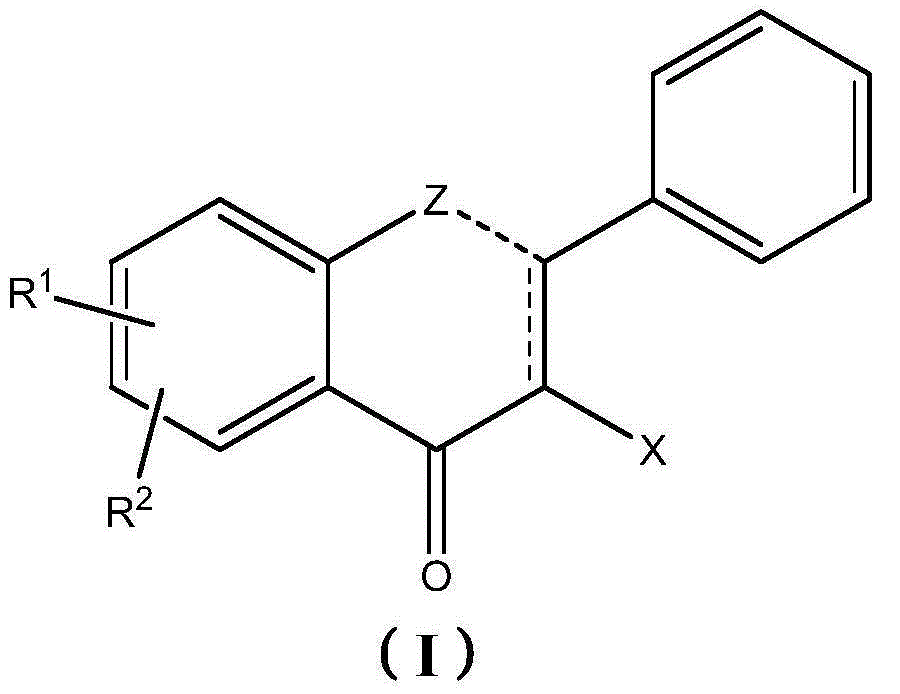
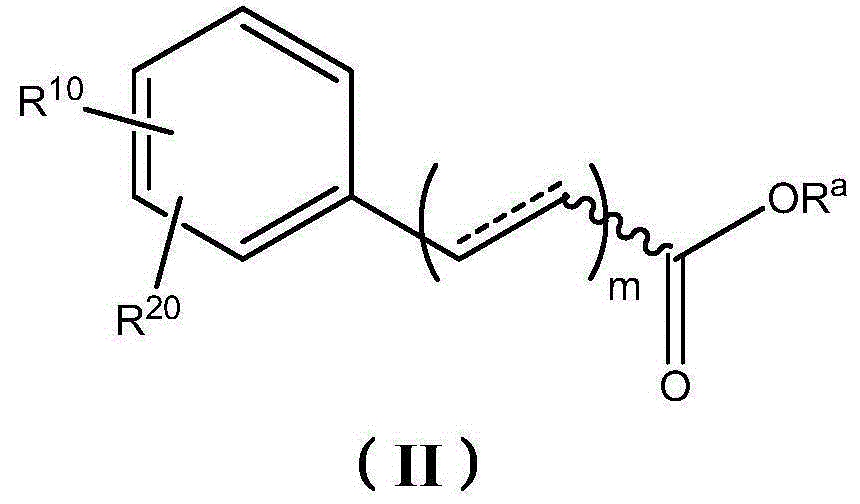
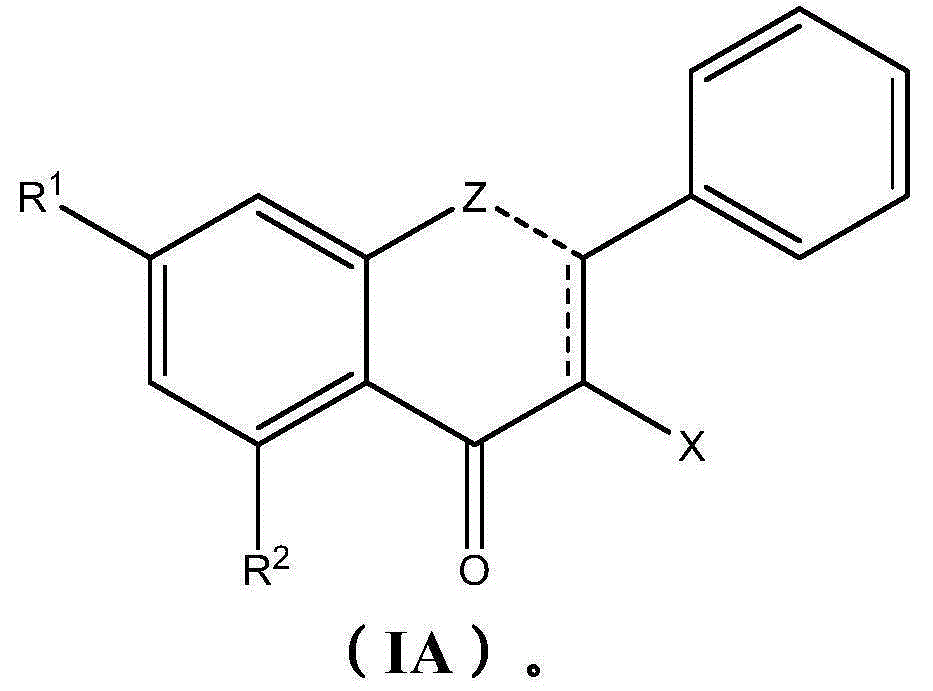
Therapeutic compositions comprising extracts of propolis and uses thereof
A composition, a technology of a pharmaceutical composition, applied in the fields of anti-gastrointestinal cancer composition, composition for treatment and prevention of gastrointestinal cancer, capable of solving problems such as no symptoms, delay in diagnosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0302] This example describes the evaluation of the anti-gastrointestinal cancer activity of propolis fractions produced by preparative chromatography. This study was performed in the colon cancer adenocarcinoma cell line DLD-1 using a proliferation assay.
[0303] Materials and methods
[0304] The test samples shown in Table 2 below were evaluated for their ability to modulate the viability and proliferation of human colorectal adenocarcinoma cells (DLD-1 ) as assessed by the MTT assay. A positive control, 5-fluorouracil (5-FU), was included in the study in addition to an unsupplemented cell control (negative control). Test samples were obtained by grading propolis tinctures.
[0305] Production of Propolis Tincture Fractions by Column Chromatography
[0306] Fractionation was performed using a glass column packed with a Merck Lichroprep CI8 reverse phase stationary phase (16 x 4 cm) that had been washed with methanol (MeOH, 200 mL) and equilibrated with 20% aqueous EtOH ...
Embodiment 2
[0357] This example describes the evaluation of the anti-gastrointestinal cancer activity of compositions of the invention compared to pure compound standards. This study was performed in the colon adenocarcinoma cell line DLD-1 using a proliferation assay as described in Example 1.
[0358] Preparative HPLC
[0359] The 20%, 30%, 60% and 90% EtOH aqueous elution fractions from Example 1 were further fractionated by preparative HPLC. The dried 20% and 30% EtOH fractions were dissolved in EtOH / H 2 O (1:1), while the 60% and 90% EtOH fractions were dissolved in pure EtOH. Each solution was chromatographically analyzed by preparative HPLC on a Phenomenex Synergi4|i,-RPMax80A250×30mmC-12 column using a Gilson321 preparative pump and an Agilent1100 series diode array detector. Injection volumes between 0.5 and 1.5 mL were used. A flow rate of 20 mL / min was used. For the 20%, 30% and 60% EtOH fractions, an initial solvent composition of 70% water (with 0.1% trifluoroacetic acid...
Embodiment 3
[0412] This example describes the evaluation of the anti-gastrointestinal cancer activity of the compound 5-phenylpenta-2,4-dienoic acid isolated from NZ-derived European-type propolis as fraction S#14 60% F7 (Table 6, Example 2). This study was performed using proliferation assays of the human colon adenocarcinoma cell line DLD-1, the human colon carcinoma cell line HCT-116, the human gastric carcinoma cell line NCI-N87, and the human esophageal squamous carcinoma cell line KYSE-30.
[0413] The general method used for Examples 3 to 13 is shown below.
[0414] Materials and methods for antiproliferative assays in gastrointestinal cancer
[0415] 5-Phenylpenta-2,4-dienoate was assessed to regulate human colorectal adenocarcinoma cell line (DLD-1), human colon cancer cell line (HCT-116), human gastric cancer cell line ( NCI-N87) and human esophageal squamous cell carcinoma cell line (KYSE-30) viability and proliferation ability. In addition to an unsupplemented cell control ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com