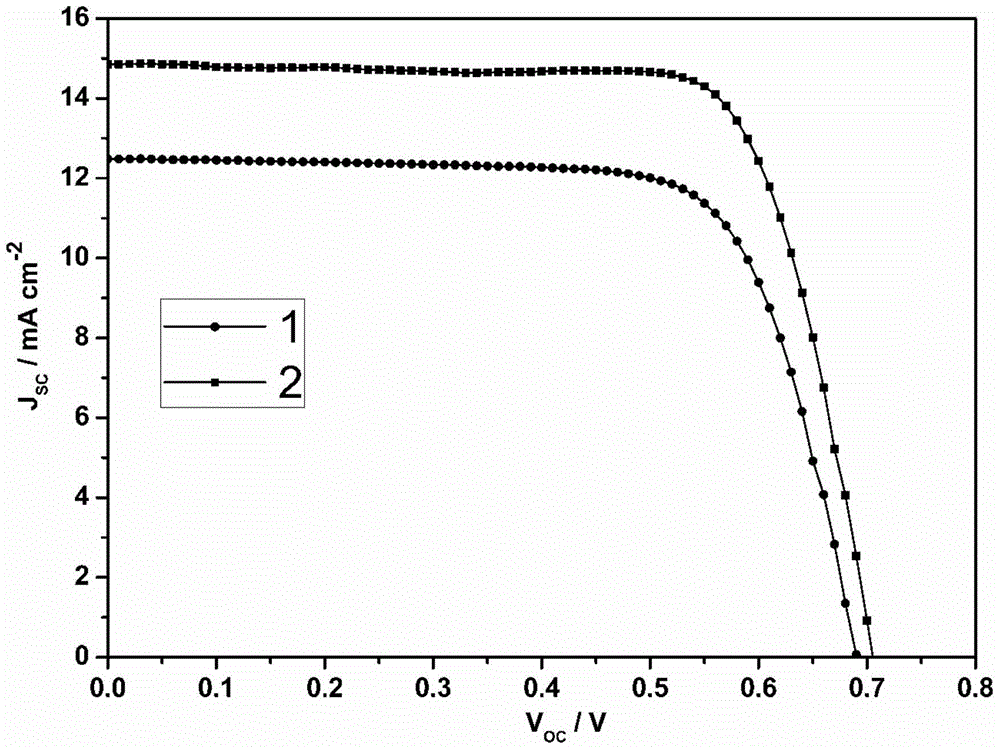
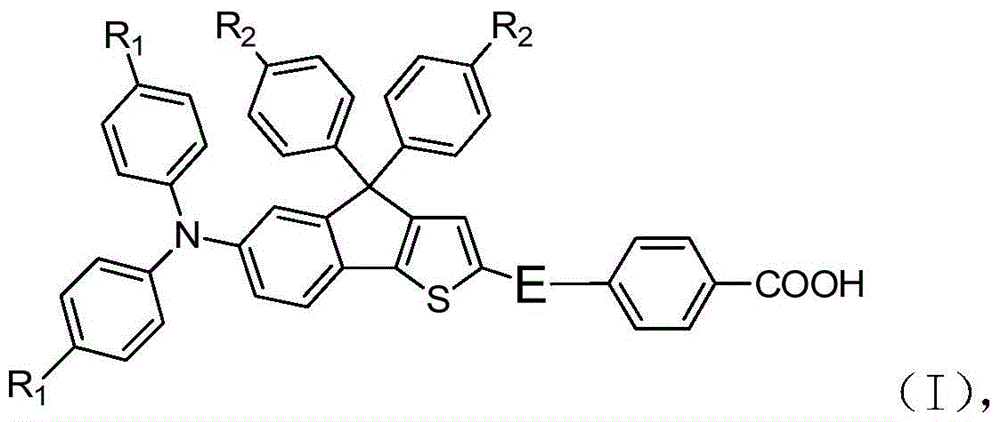
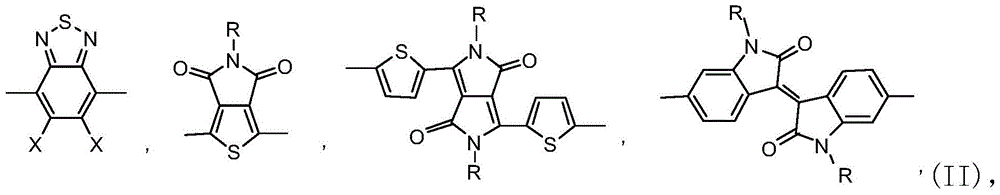
A kind of organic dye sensitizer containing triphenylamine-thiophene fluorene derivative and preparation method thereof
A technology of organic dyes and derivatives, applied in organic chemistry, triarylmethane dyes, photosensitive equipment, etc., can solve the problems of long synthetic route, low overall yield, and need to improve energy conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] An organic dye sensitizer with a chemical structure of 1, its synthetic route is as follows:
[0062]
[0063] (1) Synthesis of intermediates with chemical structural formula a:
[0064] (3.2g, 8mmol) triphenylamine borate, (1.8g, 8mmol) thiophene ester halogenated compound, (2.2g, 16mmol) K 2 CO 3 , Appropriate amount of water and tetrabutylammonium bromide, 0.1g palladium catalyst, add 80mL toluene, vacuum immediately, N 2 Protect from light, heat up to 105°C and react for 24 hours. Cool to room temperature, use CH 2 Cl 2 Extracted with water, anhydrous MgSO 4 Dry, filter and spin dry. Finally, column chromatography was performed with petroleum ether / dichloromethane as the eluent to obtain a bright yellow solid with a yield of 50%. 1 H NMR(400MHz, CDCl 3 , δ / ppm): 7.48 (d, 1H), 7.34 (d, Ar-H, 2H), 7.16 (d, 1H), 7.03-7.09 (d, Ar-H, 8H), 6.97 (d, Ar- H,2H),3.76(s,-OCH 3 ,3H),2.32(s,-CH 3 ,6H).
[0065] (2) Synthesis of intermediates with chemical structural formula b:
[0066...
Embodiment 2
[0078] The synthetic route of the organic dye sensitizer with chemical structural formula 2 is as follows:
[0079]
[0080] (1) The synthesis of the intermediate with the chemical structural formula a is the same as in Example 1
[0081] (2) The synthesis of the intermediate with the chemical structural formula b is the same as in Example 1.
[0082] (3) The synthesis of the intermediate with the chemical structural formula c is the same as in Example 1.
[0083] (4) Synthesis of intermediates with chemical structural formula f:
[0084] The (0.82g, 2.5mmol) fluorine-containing benzothiadiazole, (0.65g, 2.5mmol) borate, (0.7g, 5mmol) K 2 CO 3 , Appropriate amount of water and tetrabutylammonium bromide, 0.1g palladium catalyst, add 50mL toluene, N 2 The reaction was refluxed for 24 hours under protection. Cool to room temperature, use CH 2 Cl 2 Extracted with water, anhydrous MgSO 4 Dry, filter and spin dry. Finally, column chromatography was performed with petroleum ether / dichlorome...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com