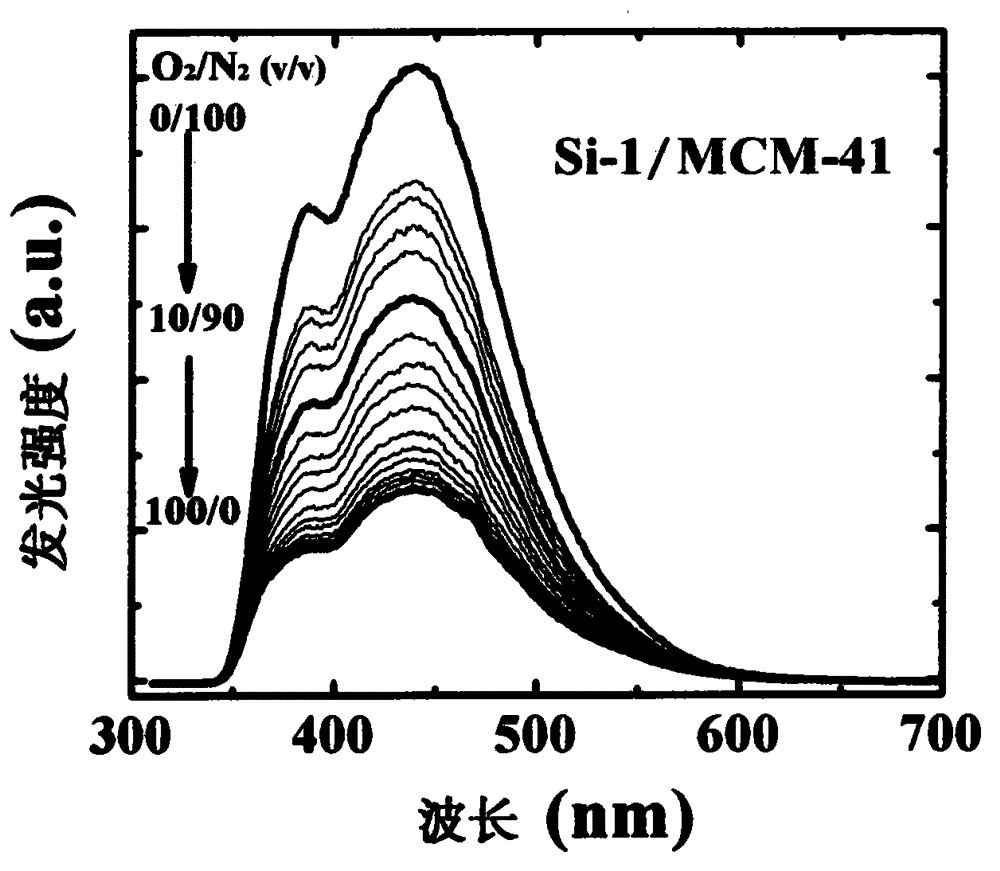
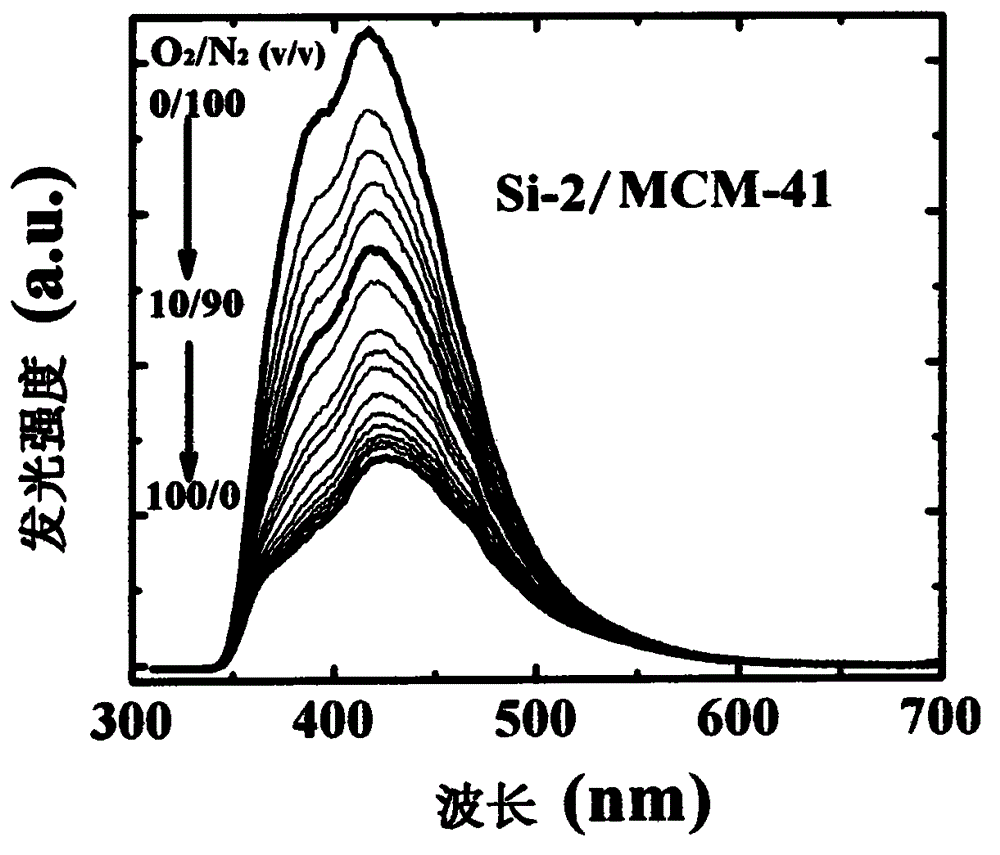
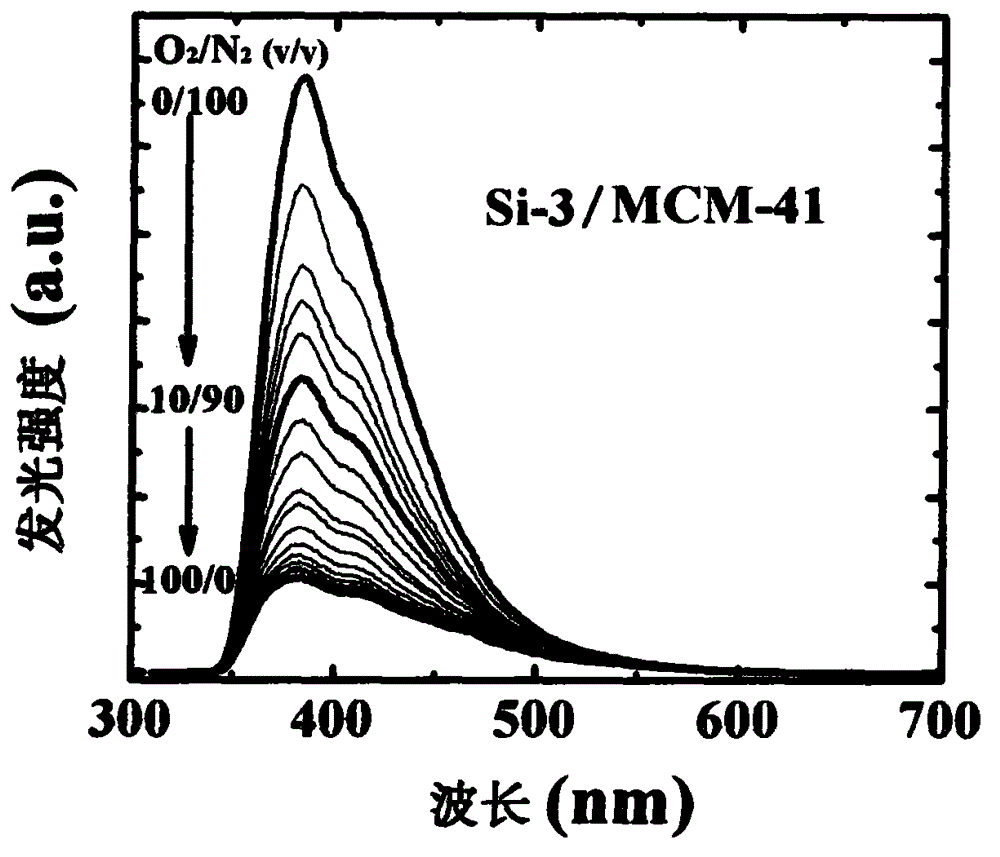
Optical oxygen sensing composite material without heavy metal element and preparation method thereof
A composite material and oxygen sensing technology, which is applied in the field of optical oxygen sensing composite materials without heavy metal elements and its preparation, can solve the problems of heavy metal element environmental pollution, expensive transition metal phosphorescent complexes, etc., and achieve simple preparation methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]Example 1: Synthesis of organosilicon compound Si-1 in structural formula (I)
[0026]
[0027] (1) At 25°C, under nitrogen protection, add 1,4-dibromobenzene (1.18g, 5.0mmol) and 20mL THF solution into a 100mL round-bottomed flask, and cool the system to -120~10°C, preferably -78°C , 2.50 mL of n-butyllithium / n-hexane solution (2.2 M, 5.5 mmol) was slowly added dropwise to the flask, and the dropwise addition time was 0.5 to 2.0 hours, preferably 1.0 hour. Trimethylchlorosilane (0.63 mL, 5.0 mmol) was added to the flask, and the stirring was continued at 70°C for 10-36 hours, preferably 24 hours. The reaction was quenched with deionized water, extracted with dichloromethane, the organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain trimethyl(4-bromophenyl)silane as a pale yellow liquid. Yield: 60.0%.
[0028] (2) Weigh trimethyl(4-bromophenyl)silane (0.50 g, 2.2 mmol) prepared in step (1) and 20 mL of...
Embodiment 2
[0029] Example 2: Synthesis of organosilicon compound Si-2 in structural formula (I)
[0030]
[0031] (1) 1,4-dibromobenzene (1.18 g, 5.0 mmol) and 20 mL of THF solution were added to a 100 mL round bottom flask at 25°C under nitrogen protection. Cool the system to -120~10℃, preferably -78℃, slowly add 2.50mL n-butyllithium / n-hexane solution (2.2M, 5.5mmol) dropwise to the flask for 0.5~2.0 hours, preferably 1.0 Hour. After adding dimethylphenylchlorosilane (0.84 mL, 5.0 mmol) to the flask, stirring was continued at 70°C for 16-32 hours, preferably 24 hours. The reaction was quenched with deionized water, extracted with dichloromethane, the organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain dimethylphenyl(4-bromophenyl)silane as a pale yellow liquid. Yield: 54.2%.
[0032] (2) Weigh dimethylphenyl (4-bromophenyl) silane (0.64 g, 2.2 mmol) prepared in step (1) and 20 mL of THF into a 100 mL round-bottome...
Embodiment 3
[0033] Embodiment 3: Synthesis of organosilicon compound Si-3 in structural formula (I)
[0034]
[0035] (1) 1,4-dibromobenzene (1.18 g, 5.0 mmol) and 20 mL of THF solution were added to a 100 mL round bottom flask at 25°C under nitrogen protection. Cool the system to -120~10℃, preferably -78℃, slowly add 2.50mL n-butyllithium / n-hexane solution (2.2M, 5.5mmol) dropwise to the flask for 0.5~2.0 hours, preferably 1.0 Hour. Methyldiphenylchlorosilane (1.05 mL, 5.0 mmol) was added to the flask, and the stirring was continued at 70°C for 10-36 hours, preferably 24 hours. The reaction was quenched with deionized water, extracted with dichloromethane, the organic phase was dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure, and the obtained solid was recrystallized with absolute ethanol to obtain methyldiphenyl(4-bromophenyl)silane White solid powder. Yield: 65.0%.
[0036] (2) Weigh methyldiphenyl (4-bromophenyl) silane (0.78 g, 2.2 mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com