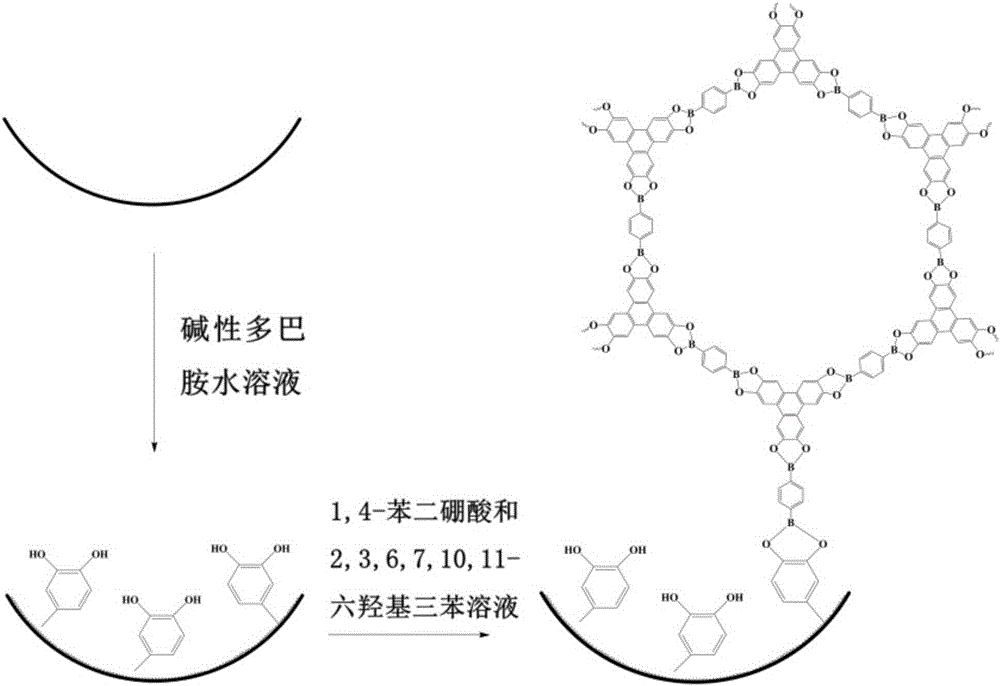
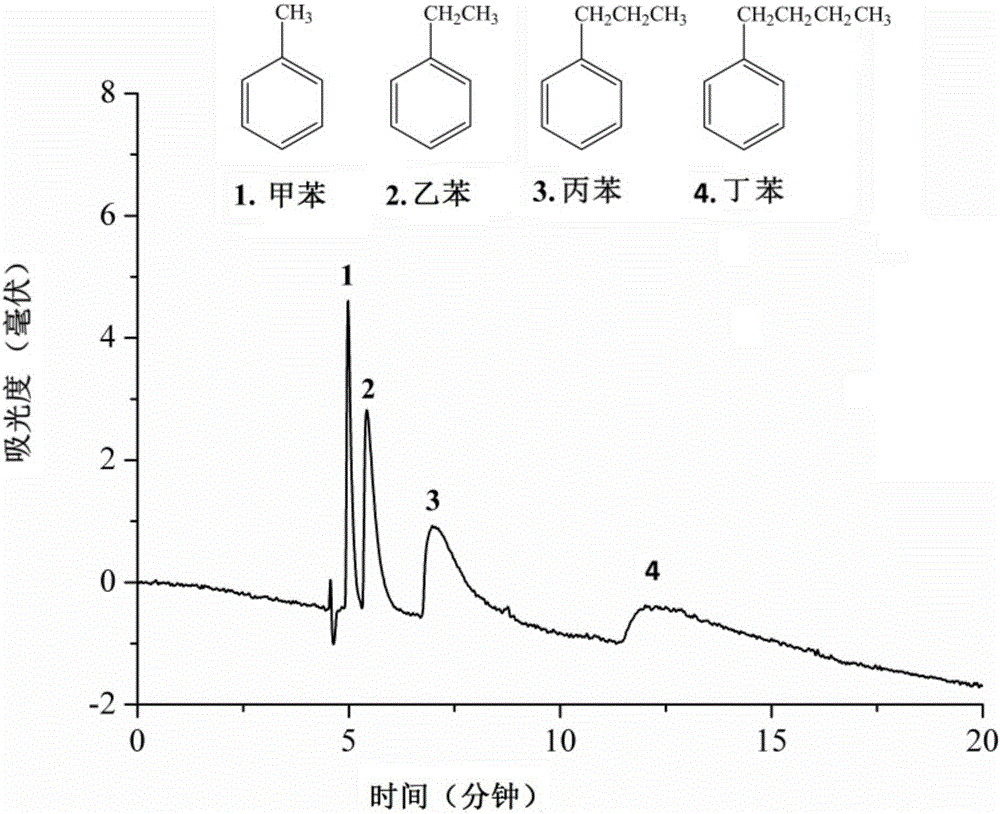
Method for fixing covalent organic framework material, and application thereof
A technology of covalent organic framework and coating, applied in the field of chromatographic column, achieves the effects of low cost, less sample consumption and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Rinse a 31cm-long quartz capillary (50μm.d.×375μmo.d.) with methanol for 2h, and dry it with nitrogen; In the quartz capillary for 10h, then rinsed with pure water and dried with nitrogen to obtain a capillary with a uniform and stable polydopamine coating on the inner wall; mix 1,4-benzenediboronic acid and 2,3,6,7,10,11- Mesitylene / 1,4-dioxane (v / v, 1:1) mixed reaction solution of hydroxytriphenyl (1,4-benzenediboronic acid and 2,3,6,7,10,11-hexa The concentration of hydroxytriphenyl is 0.75mM, 0.5mM respectively) into the quartz capillary with polydopamine coating on the inner wall for 5min, then the two ends of the quartz capillary are sealed, placed in an ultrasonic instrument for ultrasonic treatment for 1h, and heated in an oil bath at 100°C for reaction After 20 hours, rinse with methanol for 8 hours, and blow dry with nitrogen to obtain a quartz capillary with a COF-5 coating on the inner wall.
Embodiment 2
[0031] Rinse a 60cm-long polyetheretherketone capillary (PEEK tube) (50μm.d.×360μmo.d.) with methanol for 2h, and dry it with nitrogen; ,pH9) into the PEEK tube for 8 hours, rinsed with pure water, and dried with nitrogen to obtain a PEEK tube with a uniform and stable polydopamine coating on the inner wall; mix 1,4-benzenediboronic acid and 2,3,6,7, 10,11-hexahydroxytriphenyl mesitylene / 1,4-dioxane (v / v, 1:1) mixed reaction solution (1,4-benzenediboronic acid and 2,3,6,7, The concentration of 10,11-hexahydroxytriphenyl is 0.75mM, 0.75mM respectively) into the PEEK tube with polydopamine coating on the inner wall for 3min, then seal both ends of the PEEK tube, place it in an ultrasonic instrument for ultrasonic treatment for 1.5h, Heat the reaction in an oil bath at 110°C for 30 hours, rinse with methanol for 8 hours, and blow dry with nitrogen to obtain a PEEK tube with a COF-5 coating on the inner wall.
Embodiment 3
[0033] Rinse a 15cm-long polytetrafluoroethylene capillary (PTFE tube) (200μm.d.×300μmo.d.) with methanol for 2h, and dry it with nitrogen; , pH9.5) was continuously passed into the polytetrafluoroethylene capillary for 12 hours, rinsed with pure water, and dried with nitrogen to obtain a polytetrafluoroethylene capillary with a uniform and stable polydopamine coating on the inner wall; the 1,4-benzenediboronic acid and 2,3,6,7,10,11-hexahydroxytriphenyl mesitylene / 1,4-dioxane (v / v, 1:1) mixed reaction solution (1,4-benzenediboronic acid and 2,3,6,7,10,11-hexahydroxytriphenyl are 0.75mM and 0.25mM respectively) into the polytetrafluoroethylene capillary with polydopamine coating on the inner wall for 1min, and then the polytetrafluoroethylene Both ends of the ethylene capillary were sealed, placed in a sonicator for ultrasonic treatment for 2 hours, heated in an oil bath at 120°C for 10 hours, rinsed with methanol for 8 hours, and dried with nitrogen to obtain a polytetrafluor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com