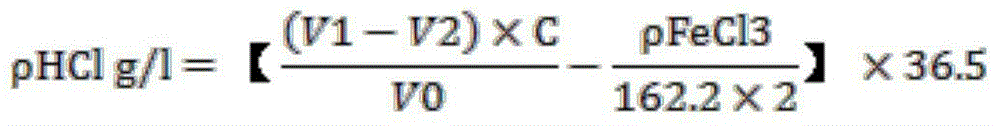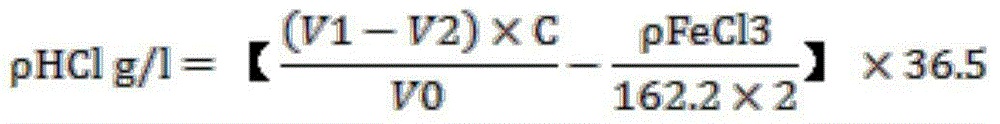Method for accurately determining content of hydrochloric acid in iron chloride corrosion solution
A technology of corrosive solution and ferric chloride, applied in the field of chemical analysis, to achieve the effect of real and reasonable calculation results, clear titration end point and narrow indication range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment is a method for measuring the content of hydrochloric acid in a superalloy corrosion solution. The technical conditions of the solution are HCl91g / L-126g / L, FeCl3242g / L-300g / L, including the following steps:
[0038] Step 101, use a pipette to accurately draw 10.00 ml of superalloy corrosion solution, place it in a 100 ml volumetric flask, dilute to the mark with distilled water, and shake well. A dilution of the superalloy corrosion solution is obtained.
[0039] Step 102, use a pipette to accurately draw 10.00 ml of the above dilution solution, and place it in a 250 ml Erlenmeyer flask. A dilution of the separated superalloy corrosion solution is obtained.
[0040] Step 103: Add 5.00 ml of ascorbic acid solution and 50 ml of distilled water to the diluent of the superalloy corrosion solution divided into 250 ml of the Erlenmeyer flask. A diluent of the superalloy corrosion solution in which iron ions are reduced to +2 valence is obtained.
[0041] S...
Embodiment 2
[0052] This embodiment is a method for measuring the content of hydrochloric acid in the metallographic corrosion solution of stainless steel. The technical conditions of the solution are HCl66g / L-76g / L, FeCl33g / L-7g / L, including the following steps:
[0053] Step 201, use a pipette to accurately absorb 10.00 ml of stainless steel metallographic crystal decay solution, place it in a 100 ml volumetric flask, dilute to the mark with distilled water, and shake well. A dilution solution of the stainless steel metallographic crystal corrosion solution is obtained.
[0054] Step 202, use a pipette to accurately draw 10.00 ml of the above dilution solution, and place it in a 250 ml Erlenmeyer flask. A dilution solution of the separated stainless steel metallographic crystal corrosion solution is obtained.
[0055] Step 203: Add 5.00 ml of ascorbic acid solution and 50 ml of distilled water to the diluted solution of the stainless steel metallographic crystal decay solution divided i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com