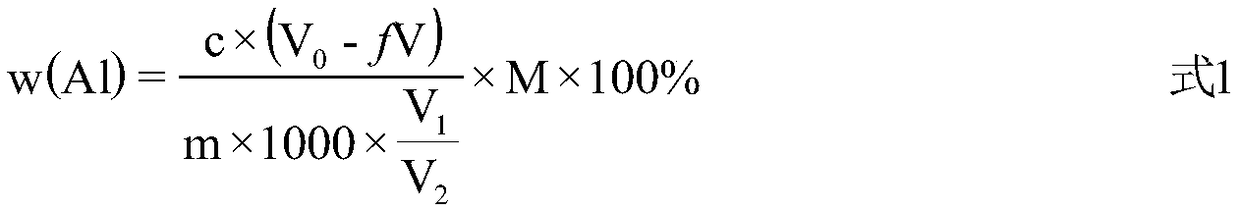Method for determining content of aluminum in aluminum-lithium alloy
A measurement method, the technology of aluminum-lithium alloy, is applied in the direction of chemical analysis by titration method and the preparation of test samples, which can solve the problems of large error and high cost, and achieve the effect of sensitive discoloration, easy identification, and avoiding the inconspicuous end point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Accurately pipette 10.00mL of 0.05139mol / L ZnO reference solution (ie V 3 ) into the Erlenmeyer flask, add 10mL of NH with pH=10 4 Cl-NH 3 ·H 2 O buffer solution, 10 drops of chrome black T indicator, titrate with EDTA standard solution until the solution changes from wine red to azure, which is recorded as the end point, and the volume of EDTA standard solution consumed is recorded (measured in parallel three times, and its average value) is respectively: 9.60mL, 9.60mL, 9.59mL, then V 4 =9.60mL; calculated by formula 2 and formula 3, c=0.05393mol / L, f=0.96.
[0067] Accurately weigh 0.4871g (i.e. m) of a bright and clean aluminum-lithium alloy into a small crucible, place it in a boiling water bath, add a small amount of water to wet it, and then add HNO with a volume ratio of 1:1 3 Make it dissolve completely; heat and stir until slightly boiled, cool to room temperature, transfer to a 100mL volumetric flask, dilute with deionized water and constant volume, shake...
Embodiment 2
[0072] In the description of embodiment 2, the determination of the concentration c of the EDTA standard solution and the volume multiple f of the EDTA standard solution equivalent to the 1mL ZnO standard solution is referred to in the embodiment 1, and only the aluminum-lithium alloy in the present embodiment is described below pretreatment and titration analysis process.
[0073] Accurately weigh 0.5021 g (i.e. m) of a bright and clean aluminum-lithium alloy in a small crucible, place it in a boiling water bath, add a small amount of water to moisten it, and then add HCl with a volume ratio of 1:1 to completely dissolve it; heat and stir until Slightly boil, cool to room temperature, transfer to a 100mL volumetric flask, dilute with deionized water and constant volume, shake well to obtain the sample.
[0074] Accurately pipette 5.00mL sample into a 250mL Erlenmeyer flask, adjust its pH to 2~3, accurately add 20.00mL EDTA standard solution, boil for a few minutes, adjust the...
Embodiment 3
[0078] In the description of embodiment 3, the determination of the concentration c of the EDTA standard solution and the volume multiple f of the EDTA standard solution equivalent to the 1mL ZnO standard solution refer to the description in embodiment 1, and only the aluminum-lithium alloy in this embodiment is described below pretreatment and titration analysis process.
[0079] Accurately weigh 0.5217g (i.e. m) of a bright and clean aluminum-lithium alloy in a small crucible, place it in a boiling water bath, add a small amount of water to moisten it, then add HCl with a volume ratio of 1:1 to completely dissolve it; heat and stir until Slightly boil, cool to room temperature, transfer to a 100mL volumetric flask, dilute with deionized water and constant volume, shake well to obtain the sample.
[0080] Accurately pipette 5.00mL sample into a 250mL Erlenmeyer flask, adjust its pH to 2~3, accurately add 25.00mL EDTA standard solution, boil for a few minutes, adjust the pH to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com