Enterovirus real-time fluorescent quantitative detection kit
A technology of real-time fluorescence quantification and detection kit, applied in the field of diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] A real-time fluorescent quantitative detection kit for enteroviruses is provided, which is used to distinguish EV71 type enteroviruses from CA16 type enteroviruses.
[0065] This enterovirus real-time fluorescent quantitative detection kit includes: upstream primer EV71F1 for amplifying EV71 enterovirus, downstream primer EV71R1 for amplifying EV71 enterovirus, and EV71 enterovirus detection Probe EV71P1, the two ends of the probe EV71P1 are respectively combined with a first fluorescent group and a first fluorescent quencher; the upstream primer CA16F2 for amplifying CA16 type enterovirus is used for amplifying CA16 type enterovirus The downstream primer CA16R2 of the virus is used to detect the probe CA16P2 of CA16 enterovirus. The two ends of the probe CA16P2 are respectively combined with a second fluorescent group and a second fluorescent quencher. The first fluorescent group and the second fluorescent The groups are different; the upstream primer F3 used to amplif...
Embodiment 2
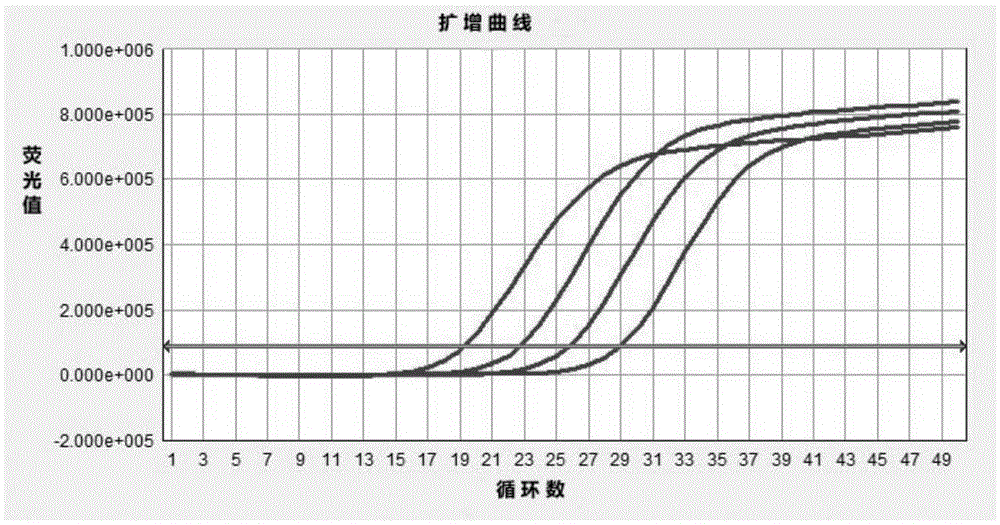
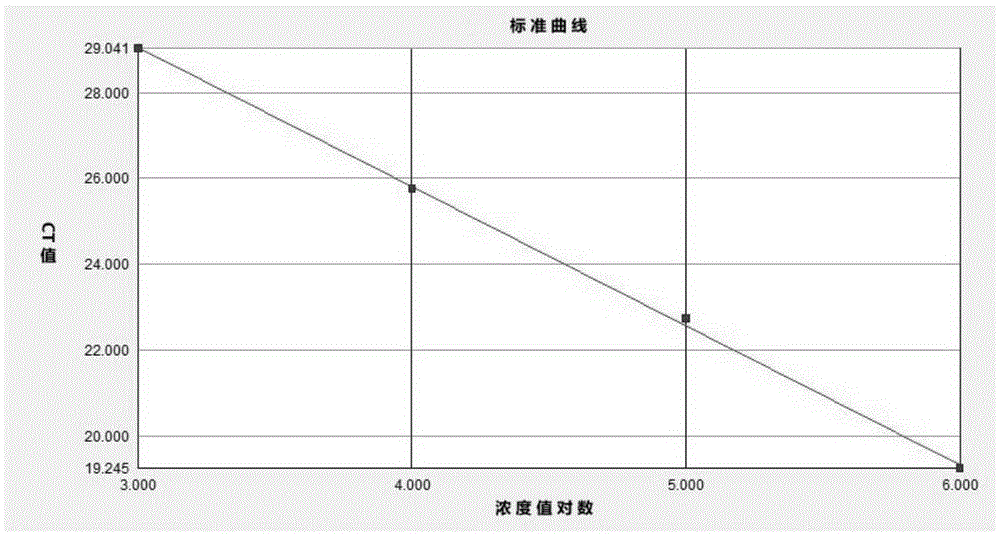
[0068] Embodiment 2 The minimum detection amount of the kit
[0069] A commercial nucleic acid RNA extraction kit OMEGAE.Z.N.A.TotalRNAKitI (OMEGA, R6834-01) was used to extract viral RNA from virus isolation cultures to obtain samples.
[0070] The concentration of nucleic acid in the sample was measured with a UV-visible photometer, and the viral nucleic acid in the sample was sequentially diluted to 1.0×10 with sterile deionized water. 6 copy, 1.0×10 5 copy, 1.0×10 4 copy, 1.0×10 3 copy, 1.0×10 2 copy, 10 copies and 1 copy.
[0071] After detection and quantification, the minimum detection amount of the enterovirus real-time fluorescence quantitative detection kit is 10 copies.
Embodiment 3
[0072] Accuracy and specificity of embodiment 3 kits
[0073] The isolated cultures of EV71 enterovirus and CA16 enterovirus were mixed as the experimental group, and hepatitis B virus (HBV), hepatitis C virus (HCV), Epstein-Barr virus (EBV) and human cytomegalovirus were selected. (HCMV), Mycoplasma pneumoniae (MP) and Treponema pallidum (TP) isolated cultures were mixed as a control group.
[0074] Among them, the selected hepatitis B virus (HBV), hepatitis C virus (HCV), Epstein-Barr virus (EBV), human cytomegalovirus (HCMV), Mycoplasma pneumoniae (MP) and Treponema pallidum (TP) were tested by corresponding reagents. All the cassettes were positive and confirmed by sequencing as corresponding pathogenic microorganisms.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com