Method for synthesizing pyridino-imidazole compounds
A technology for pyridoimidazole and compound, which is applied in the field of synthesizing pyridoimidazole compounds, can solve the problems of difficult synthesis, harsh reaction conditions, poor stability of raw materials, etc., and achieves the effects of simple operation process, easy acquisition and easy amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
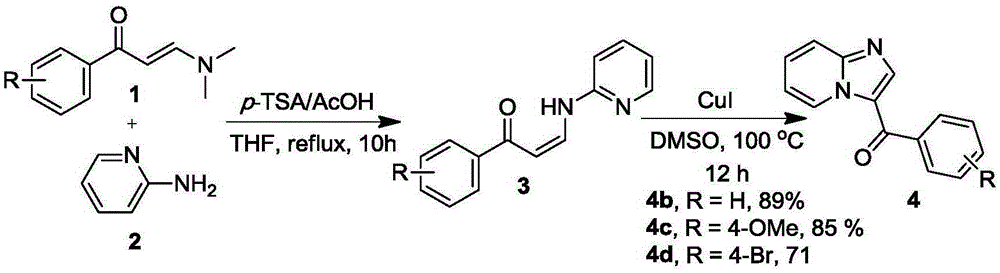
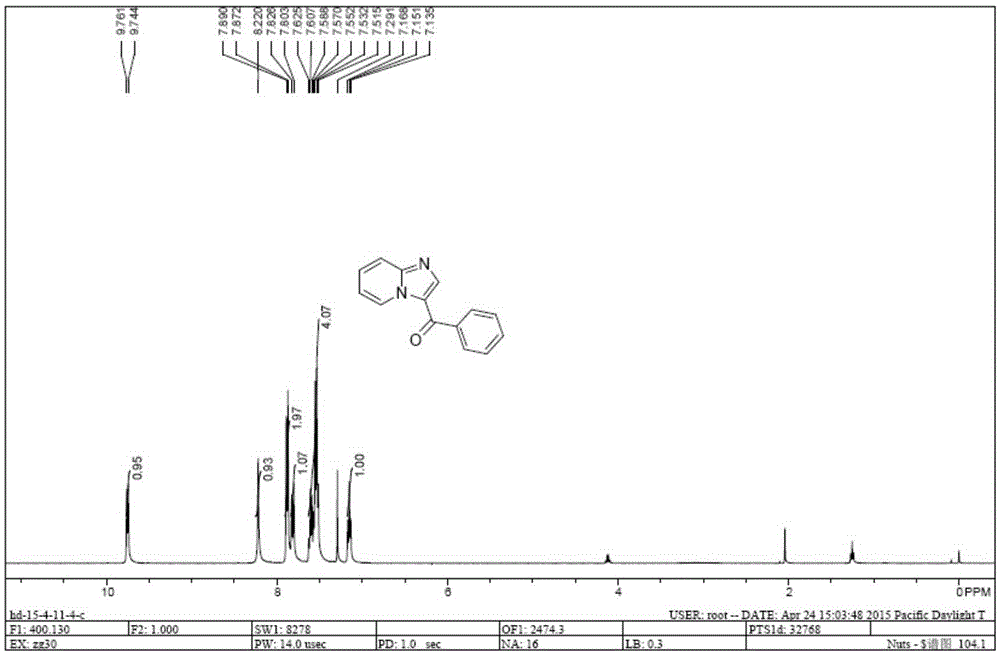
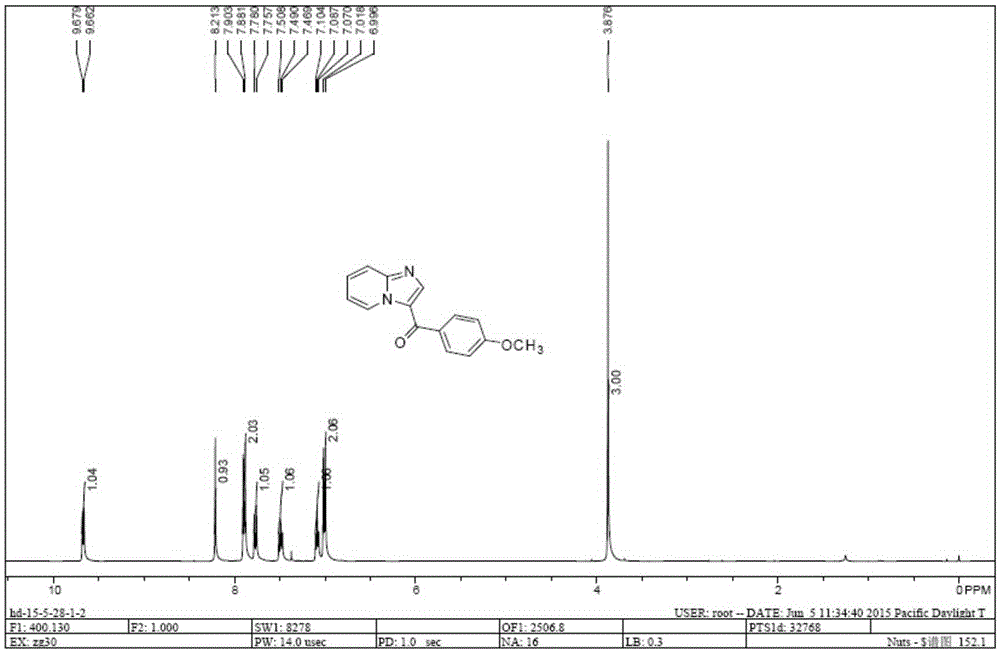
[0011] The present invention is achieved in this way: N,N-dimethylamino-substituted enaminone 1 (2mmol), 2-aminopyridine 2 (2.4mmol), p-toluenesulfonic acid (p-TSA) 2mmol and acetic acid (AcOH ) 2mmol and tetrahydrofuran (6mL) were placed in the 25mL round-bottomed flask of the condenser tube and magneton, and the resulting mixture was heated under the reflux condition of condensed water for 10h and then stopped. The ester and petroleum ether (V / V=1:10) were purified to obtain the solid enaminone intermediate 3 containing NH structure. The resulting solid 3 (0.3mmol), 0.06mmol of ketone iodide and 2mL of DMSO were placed in a 25mL round-bottomed flask equipped with a condenser tube and a magnet, and the resulting mixture was heated and stirred at 100°C for 12h in the atmosphere. 10 mL of water was added, and the condensation mixture was extracted three times with 30 mL of ethyl acetate. The organic phases were combined and dried over anhydrous sodium sulfate, the solid was fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com