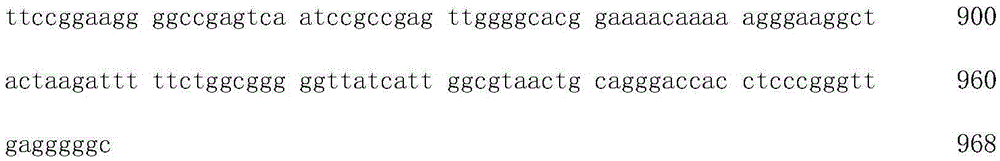Gene sequence capable of preventing gradual inactivation of promoter in subculture and applications of gene sequence
A technology of gene sequence and culture medium, which is applied in the field of gene sequence to prevent the gradual inactivation of promoters during subculture, can solve the problems affecting the yield of monoclonal antibodies, and can not achieve high yield and low cost of monoclonal antibodies, so as to reduce production The effect of high cost and high output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The genomic DNA of CHO cells was incompletely digested with EcoRI endonuclease, and the resulting fragments were randomly connected to the expression vector for screening:
[0049] The screening method is:
[0050] 1) Put 0.5×10 5 ~2×10 5 (A) CHO cells were seeded on a culture plate and added to complete medium without antibiotics;
[0051] 2) Prepare the complex: Dilute each DNA and Lipofectamine2000 obtained by incomplete digestion of CHO cells in a serum-free and antibiotic-free culture medium, mix them, incubate at room temperature for 5 minutes, mix the two, mix well, and incubate at room temperature 20 minutes;
[0052] 3) Aspirate the culture medium from the culture plate and wash the cells with serum-free medium;
[0053] 4) Add the compound evenly to the culture plate;
[0054] 5) Put the culture plate into the incubator and incubate for 4-6 hours;
[0055] 6) Observe the expression of the transferred gene after 24 to 48 hours: take the culture supernatant and detect the ...
Embodiment 2
[0058] Insert the aforementioned sequence into the vector (pCOCKCMVwt, synthesized by the company itself, the sequence is from addgene): 0.5~2×10 1 day before transfection 5 Adalimumab-producing CHO cells were seeded on a 24-well culture plate, and 500 μl of complete medium without antibiotics was added to ensure that the cell confluence reached 90-95% during transfection. Dilute 0.8μg plasmid DNA (pCOCKCMVwt, synthesized by our company, sequence derived from addgene) in 50μl serum-free and antibiotic-free culture medium and mix gently; dilute 2μl Lipofectamine2000 in 50μl serum- and antibiotic-free culture medium and mix gently Evenly, incubate at room temperature for 5 minutes. Note: It must be done within 25 minutes; after 5 minutes, mix them and mix gently, and incubate at room temperature for 20 minutes. Aspirate the culture medium from the culture plate, and wash the cells twice with serum-free medium. Add the complex (100 μl total volume) to the culture wells, shake the...
Embodiment 3
[0063] Insert the aforementioned sequence into the vector (pCOCKCMVwt, synthesized by the company itself, the sequence is from addgene): 0.5~2×10 1 day before transfection 5 Adalimumab-producing CHO cells were seeded on a 24-well culture plate, and 500 μl of complete medium without antibiotics was added to ensure that the cell confluence reached 90-95% during transfection. Dilute 0.8μg plasmid DNA (pCOCKCMVwt, synthesized by our company, sequence derived from addgene) in 50μl serum-free and antibiotic-free culture medium and mix gently; dilute 2μl Lipofectamine2000 in 50μl serum- and antibiotic-free culture medium and mix gently Evenly, incubate at room temperature for 5 minutes. Note: It must be done within 25 minutes; after 5 minutes, mix them and mix gently, and incubate at room temperature for 20 minutes. Aspirate the culture medium from the culture plate, and wash the cells twice with serum-free medium. Add the complex (100 μl total volume) to the culture wells, shake the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com