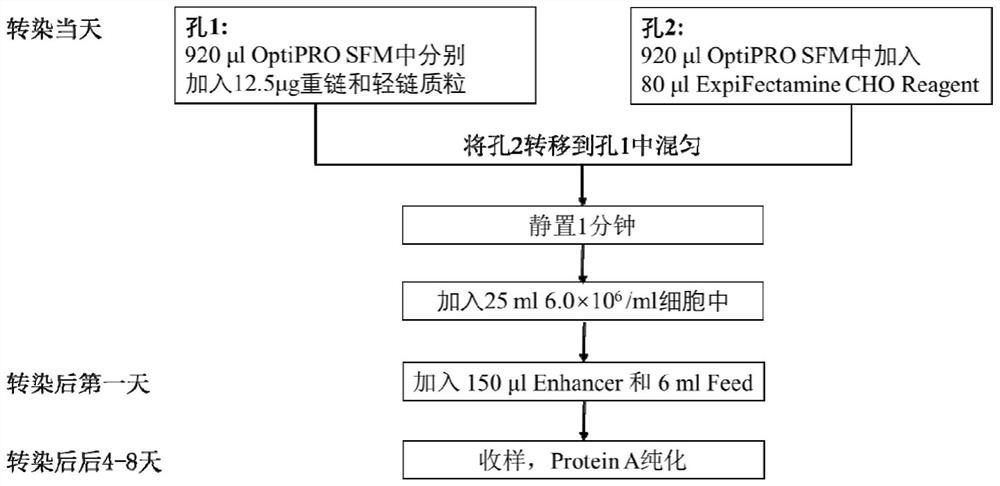
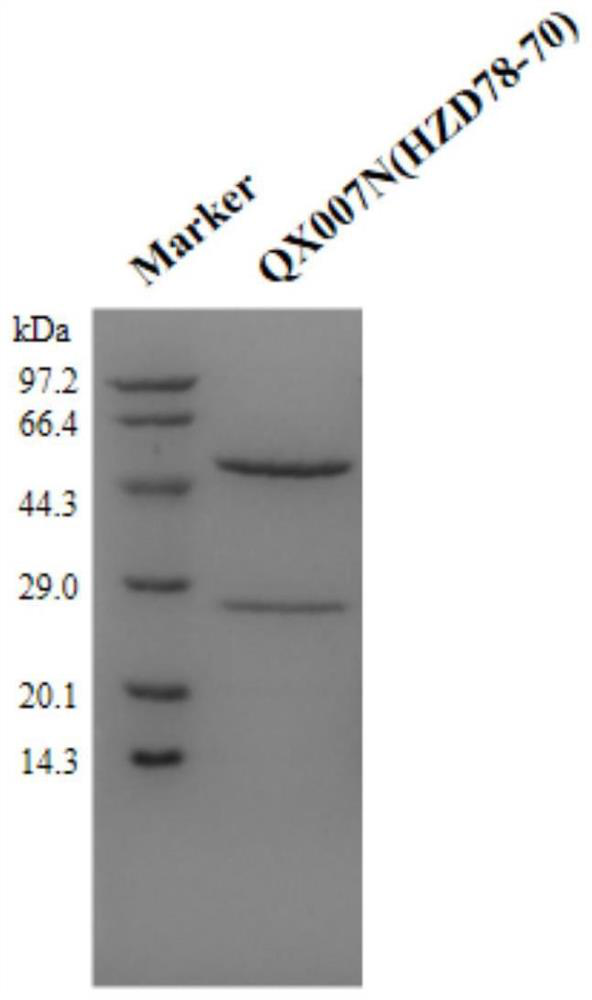
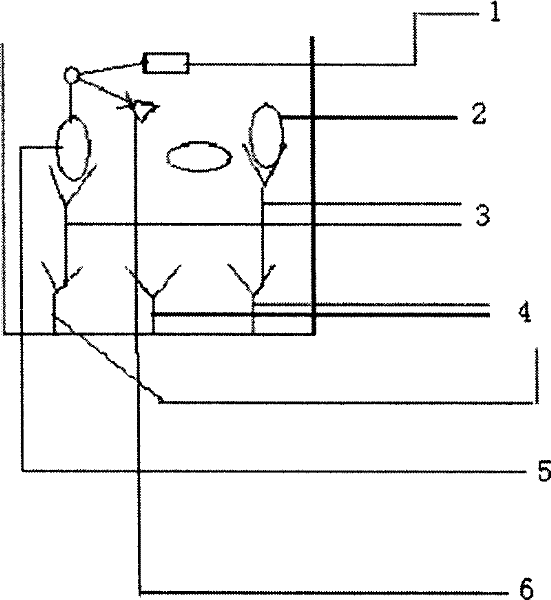
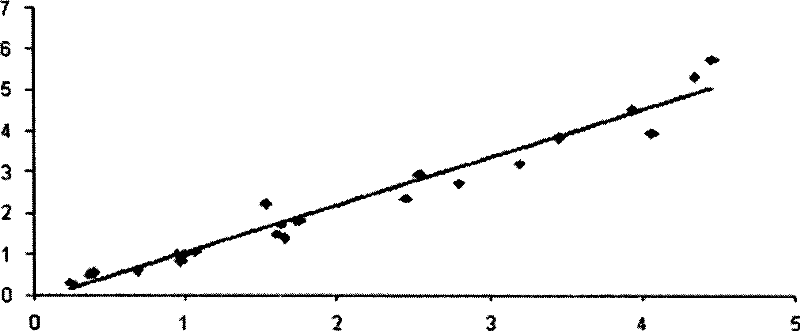
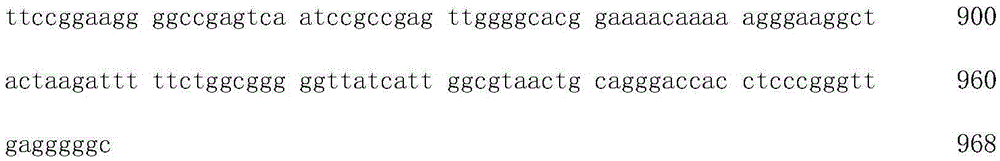Patents
Literature
40 results about "Monoclonal antibody production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
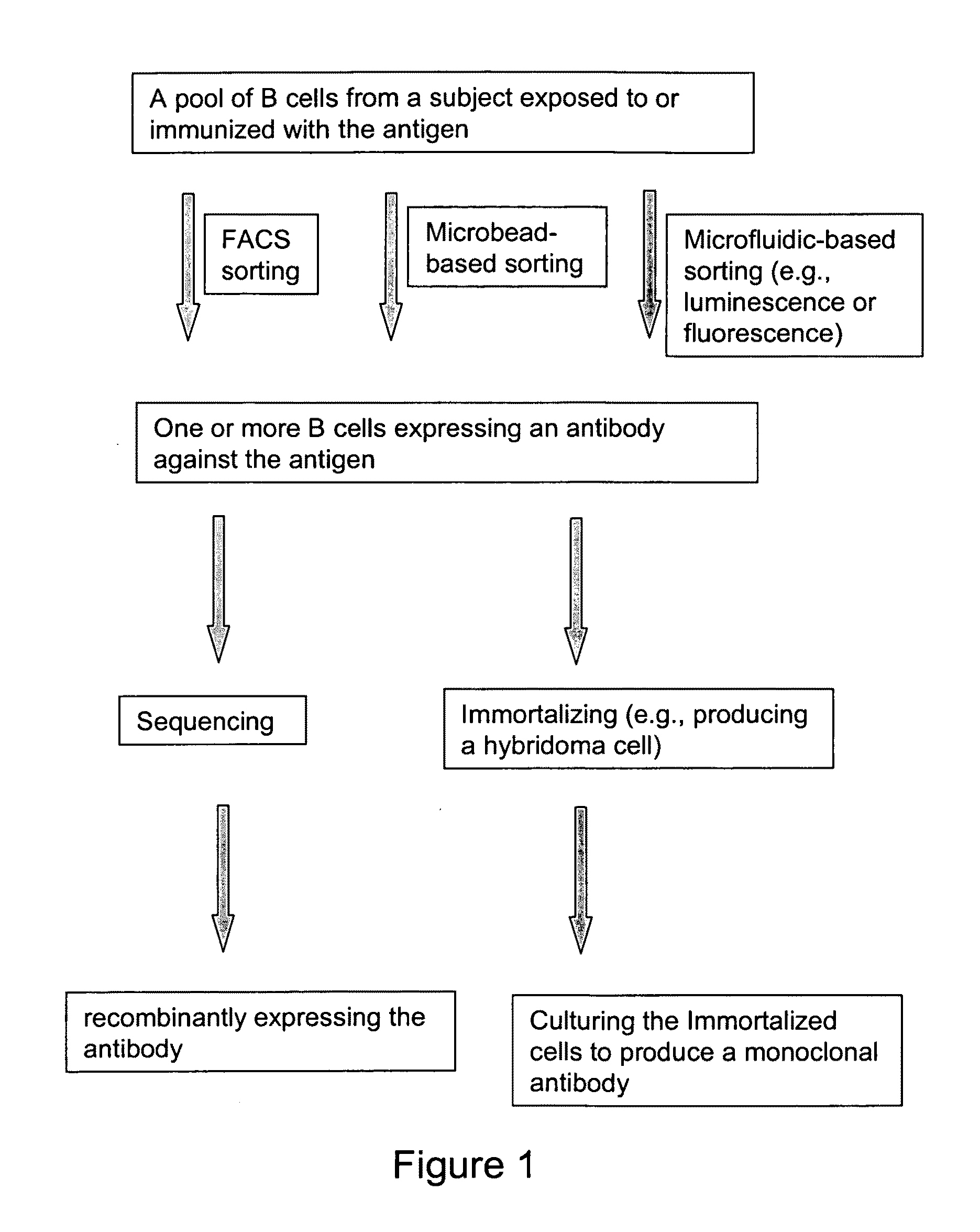
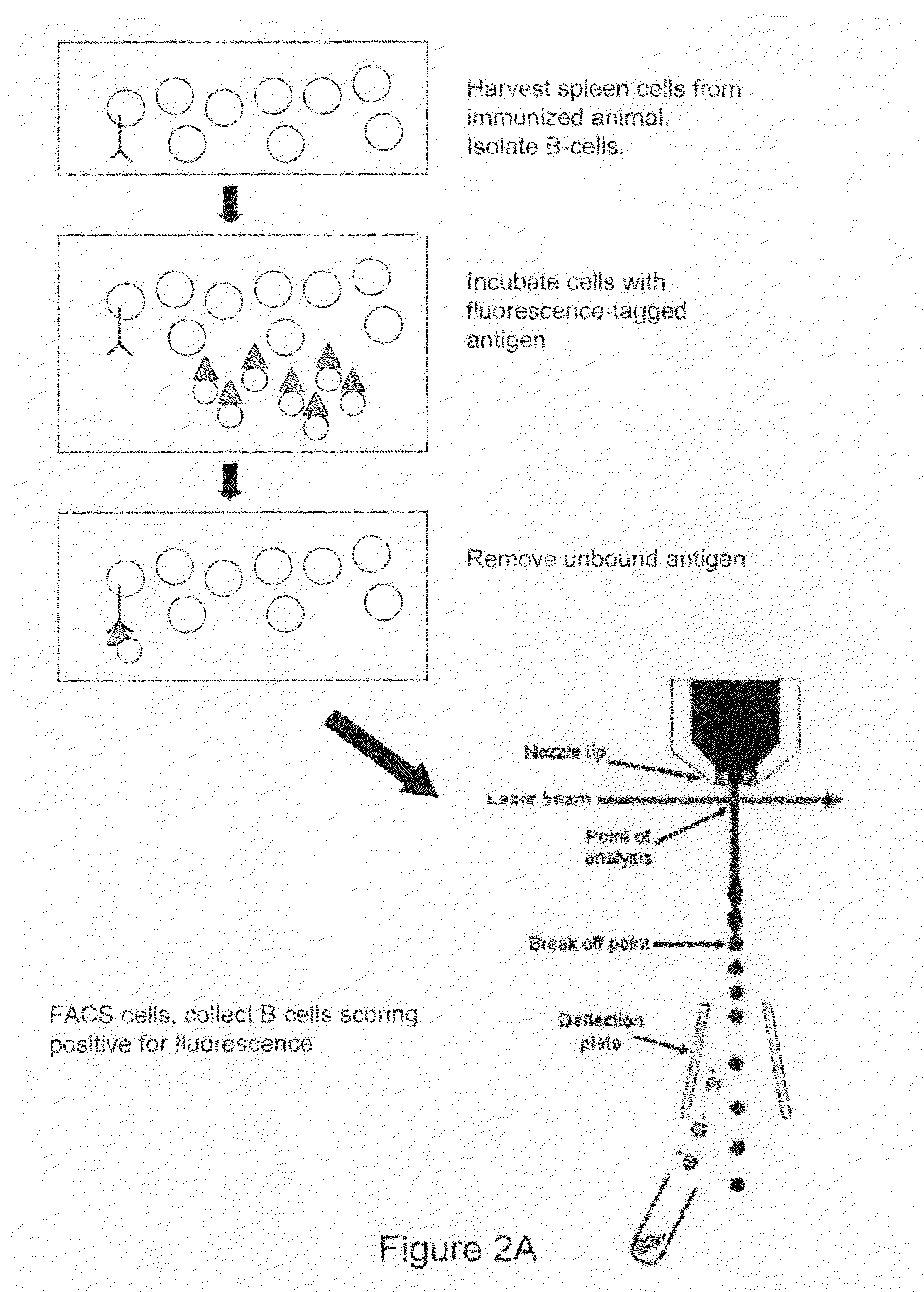
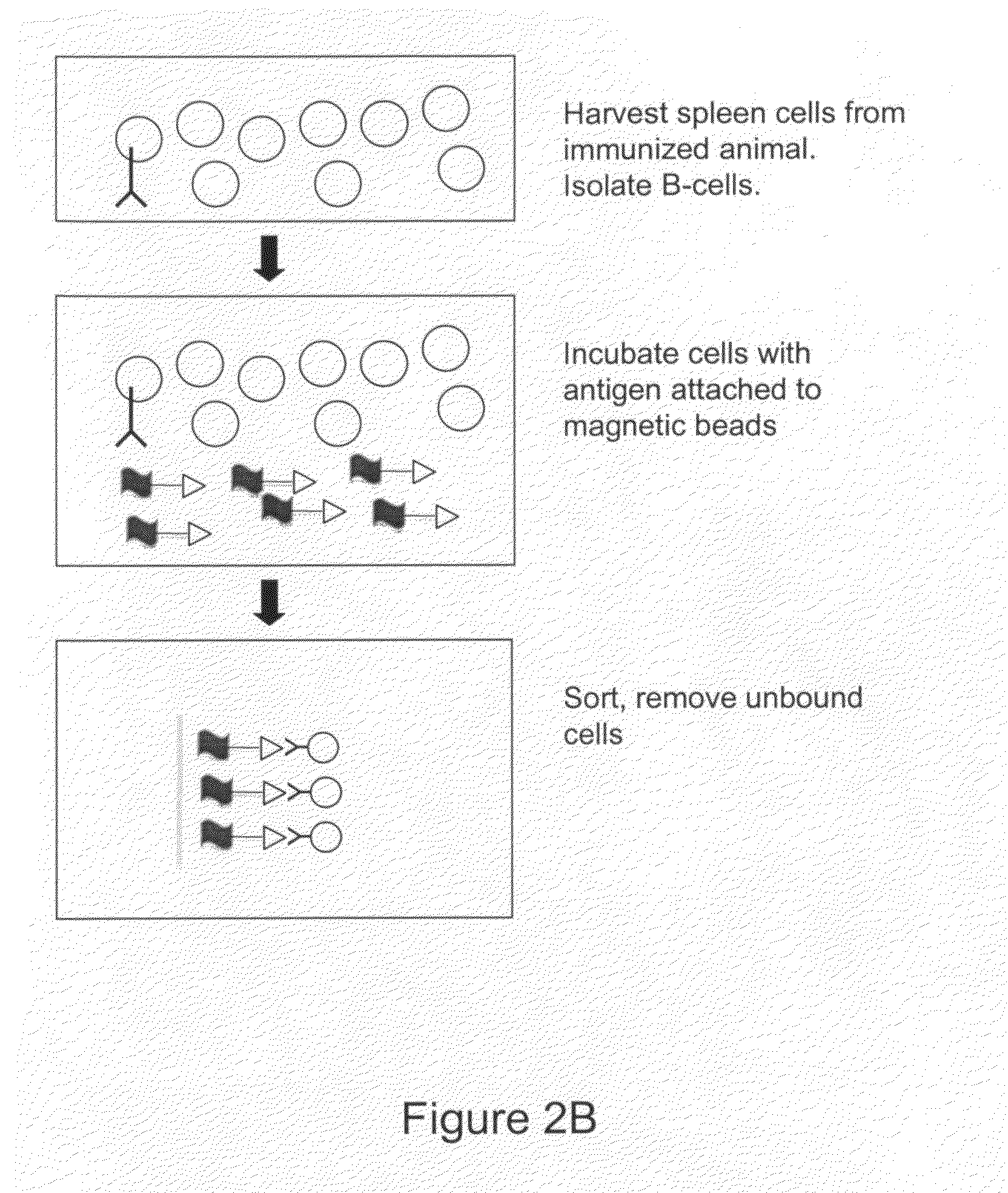
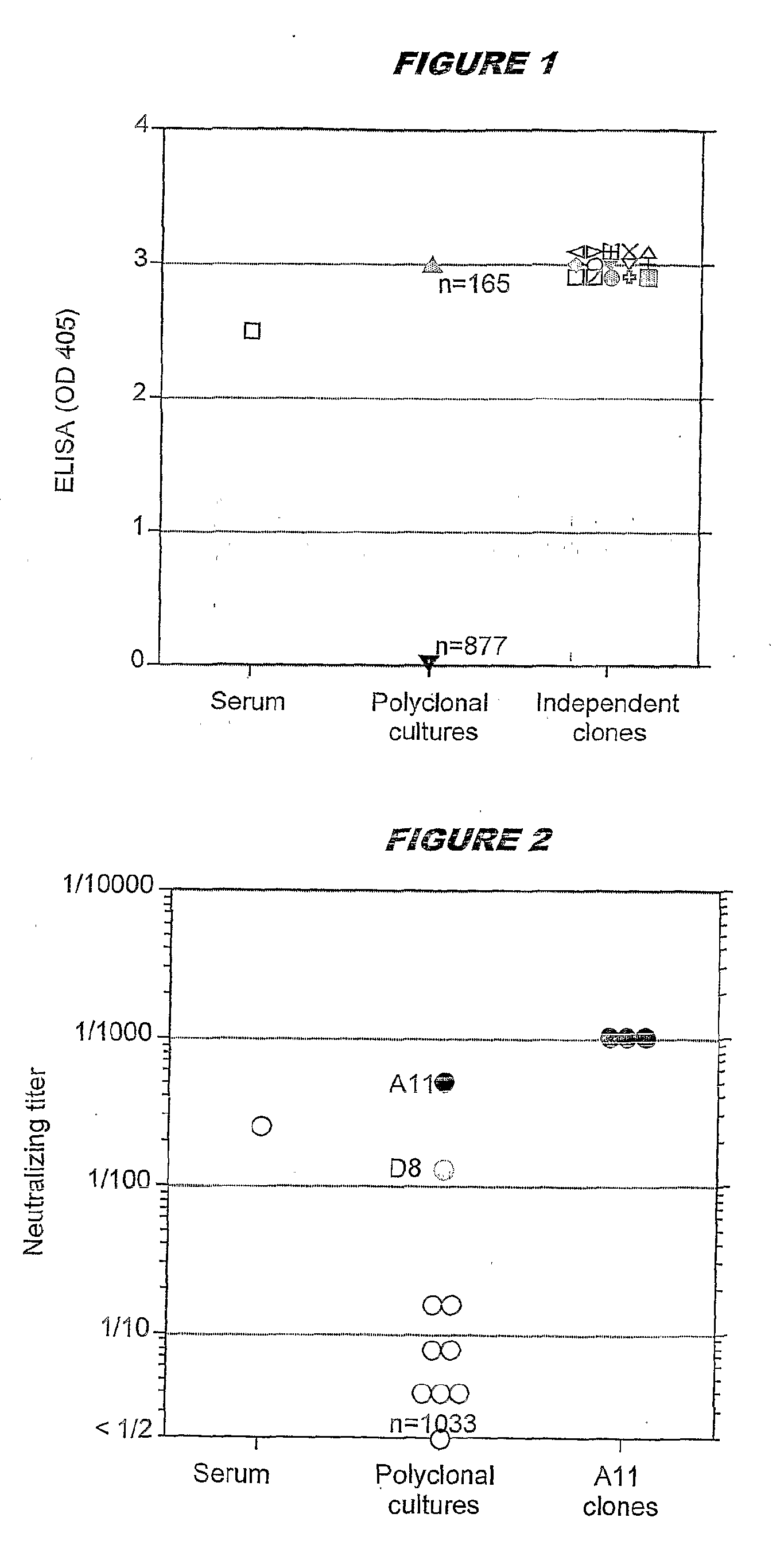
Devices and methods for immunoglobulin production
InactiveUS20100086919A1Improve efficiencyLow costMicrobiological testing/measurementMutant preparationIntravenous gammaglobulinHybridoma cell
The present invention relates to devices and methods that are useful for screening and isolating cells that express a desired immunoglobulin. The present invention further relates to devices and methods that are useful in producing monoclonal antibodies from non-immortal cells (such as B cells) or immortalized cells (such as hybridoma cells). The devices and methods disclosed herein significantly improve the efficiency for monoclonal antibody production.
Owner:CHINA MOLECULAR
Monoclonal antibody production by ebv transformation of b cells
ActiveUS20100021470A1Improve efficiencyRapid isolationImmunoglobulins against bacteriaImmunoglobulins against virusesEpstein bar virusLymphocyte
A method for producing a clone of an immortalised human B memory lymphocyte, comprising the step of transforming human B memory lymphocytes using Epstein Barr Virus (EBV) in the presence of a polyclonal B cell activator. The method is particularly useful in a method for producing a clone of an immortalised human B memory lymphocyte capable of producing a human monoclonal antibody with a desired antigen specificity, comprising the steps of: (i) selecting and isolating a human memory B lymphocyte subpopulation; (ii) transforming the subpopulation with Epstein Barr Virus (EBV) in the presence of a polyclonal B cell activator; (iii) screening the culture supernatant for antigen specificity; and (iv) isolating an immortalised human B memory lymphocyte clone capable of producing a human monoclonal antibody having the desired antigen specificity.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE
Preparing method for recombinant human anti-rabies monoclonal antibodies
Owner:NCPC NEW DRUG RES & DEV
Improved process for production of monoclonal antibodies
InactiveUS20160215319A1Reduce the temperatureSpeed up the processImmunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against growth factorsGlycanMammalian cell
The present invention provides for an improved process to obtain substantial amount of monoclonal antibodies with desired profile of charged variants. The process involves initially culturing e mammalian cells at a suitable temperature and subsequently reducing the temperature and optionally by simultaneous addition of suitable amino acid(s) during production of the desired molecule. The present invention provides also provides for an antibody having desired profile of glycans prepared with said with improved process.
Owner:CADILA HEALTHCARE LTD
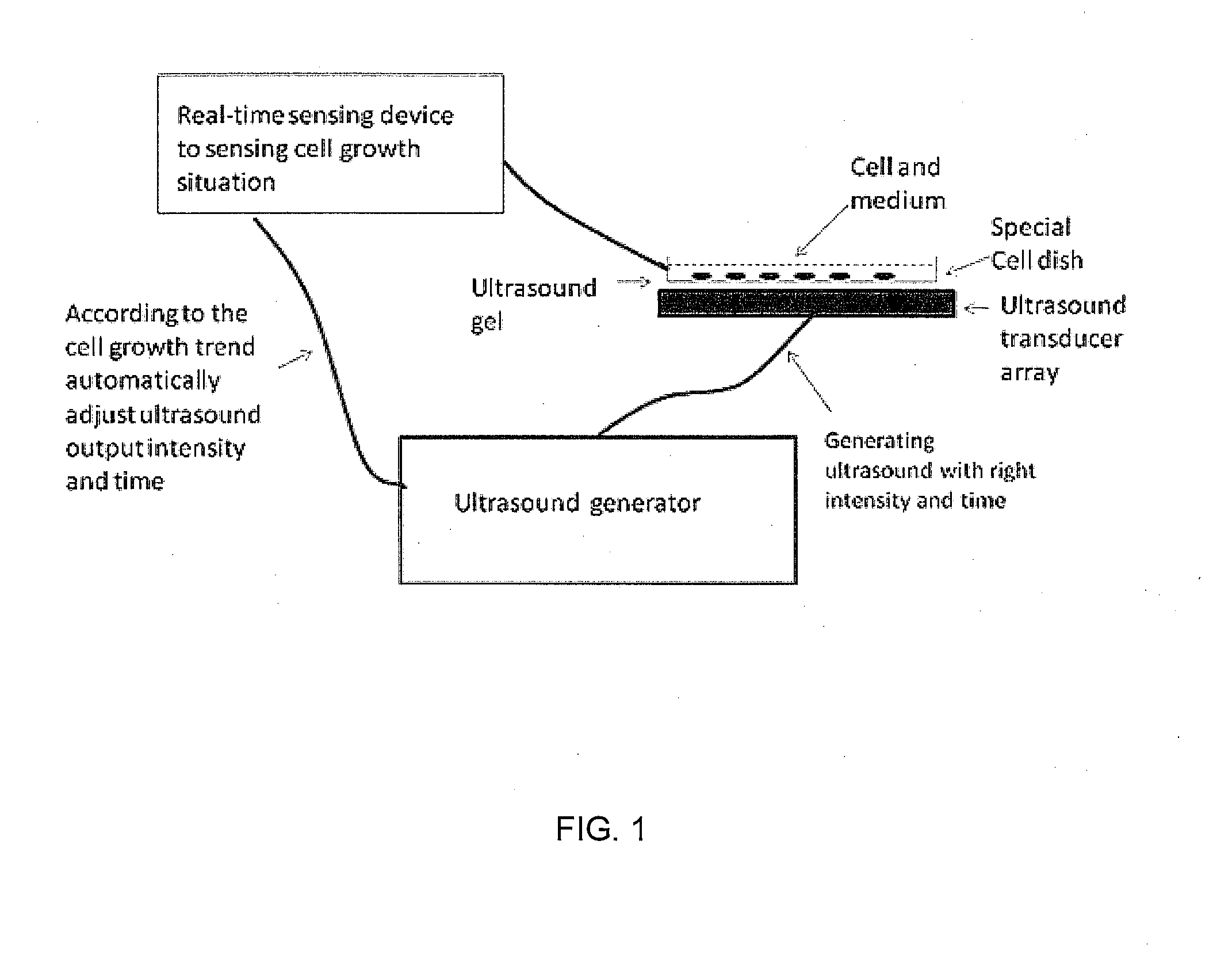
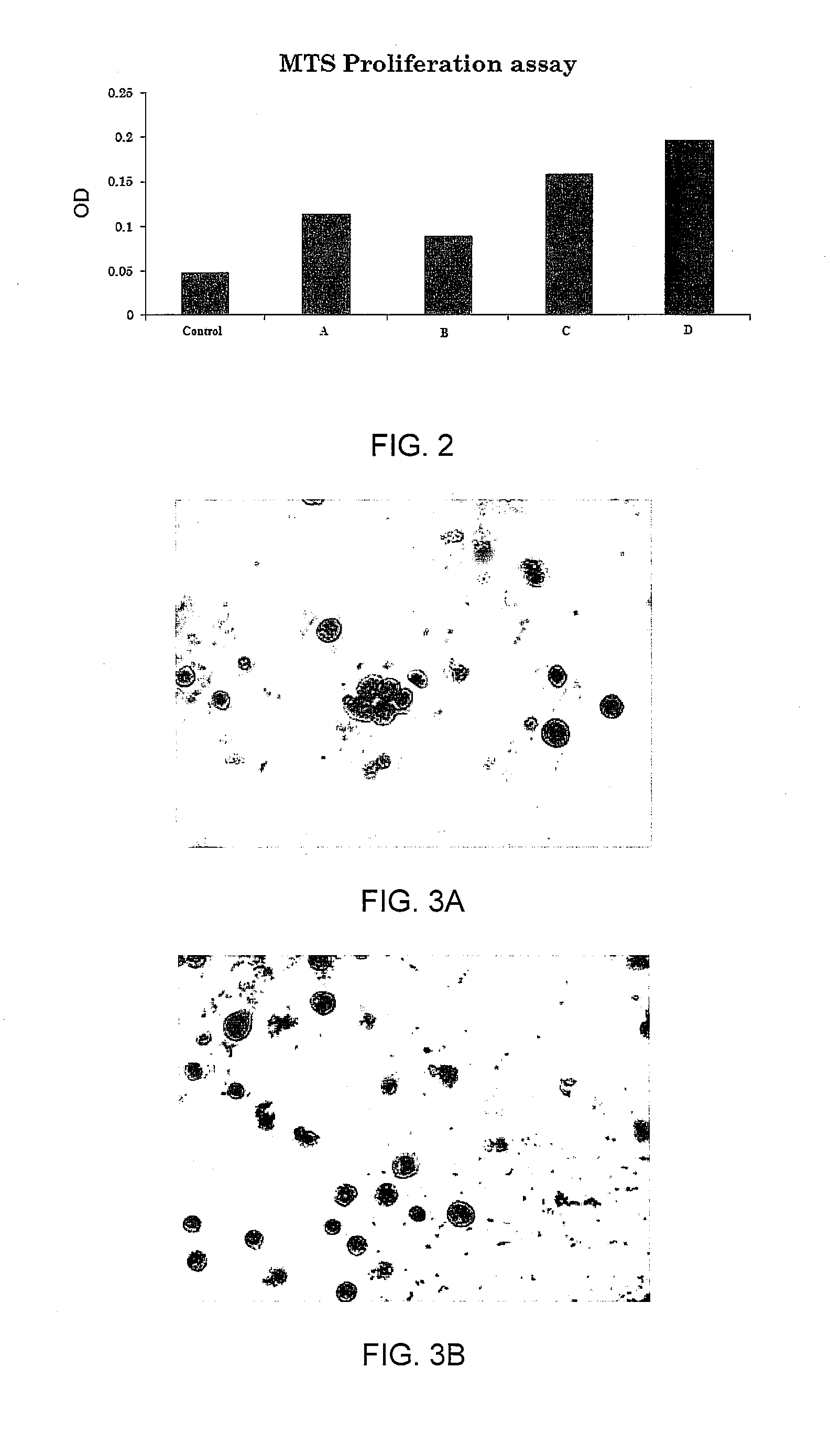
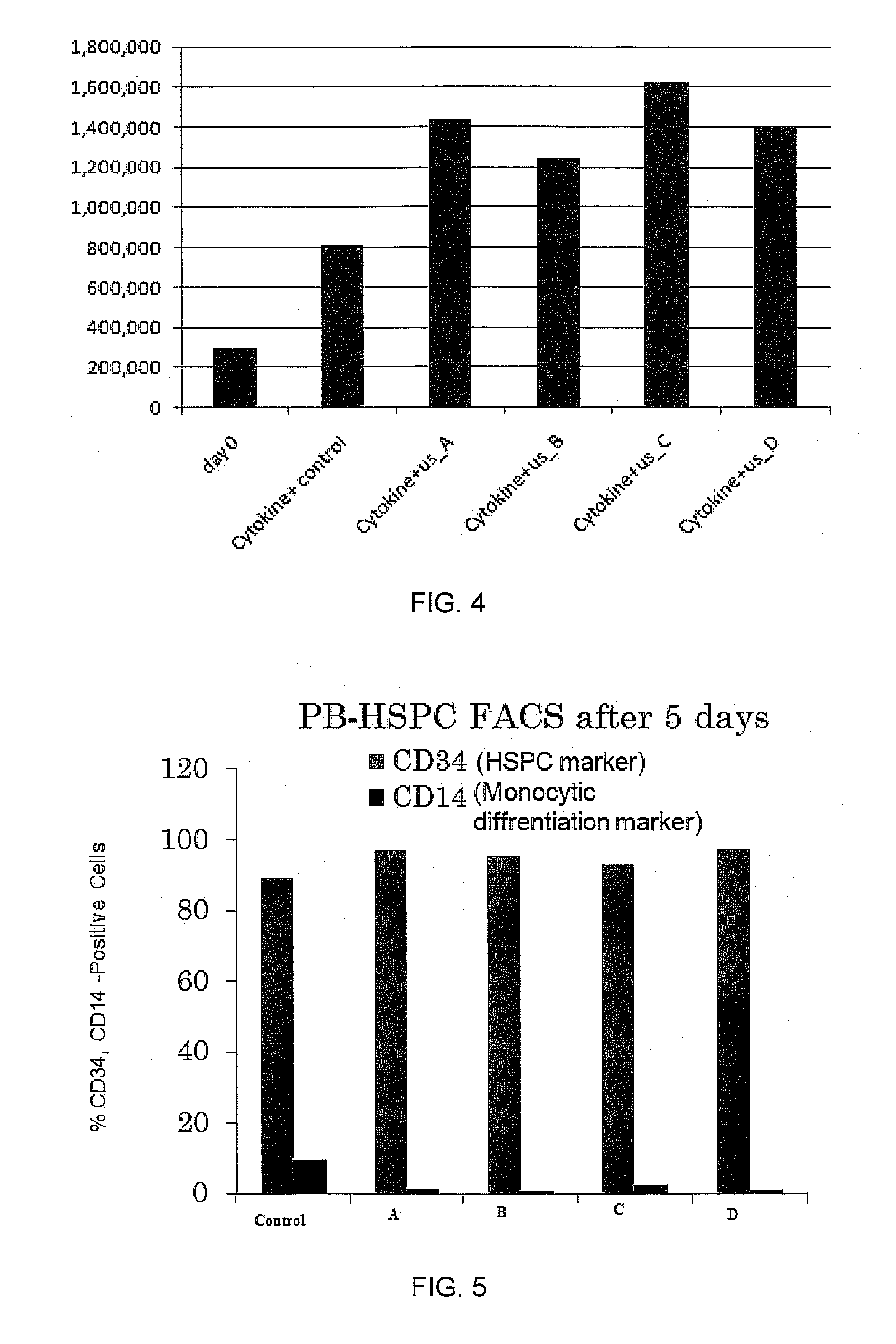
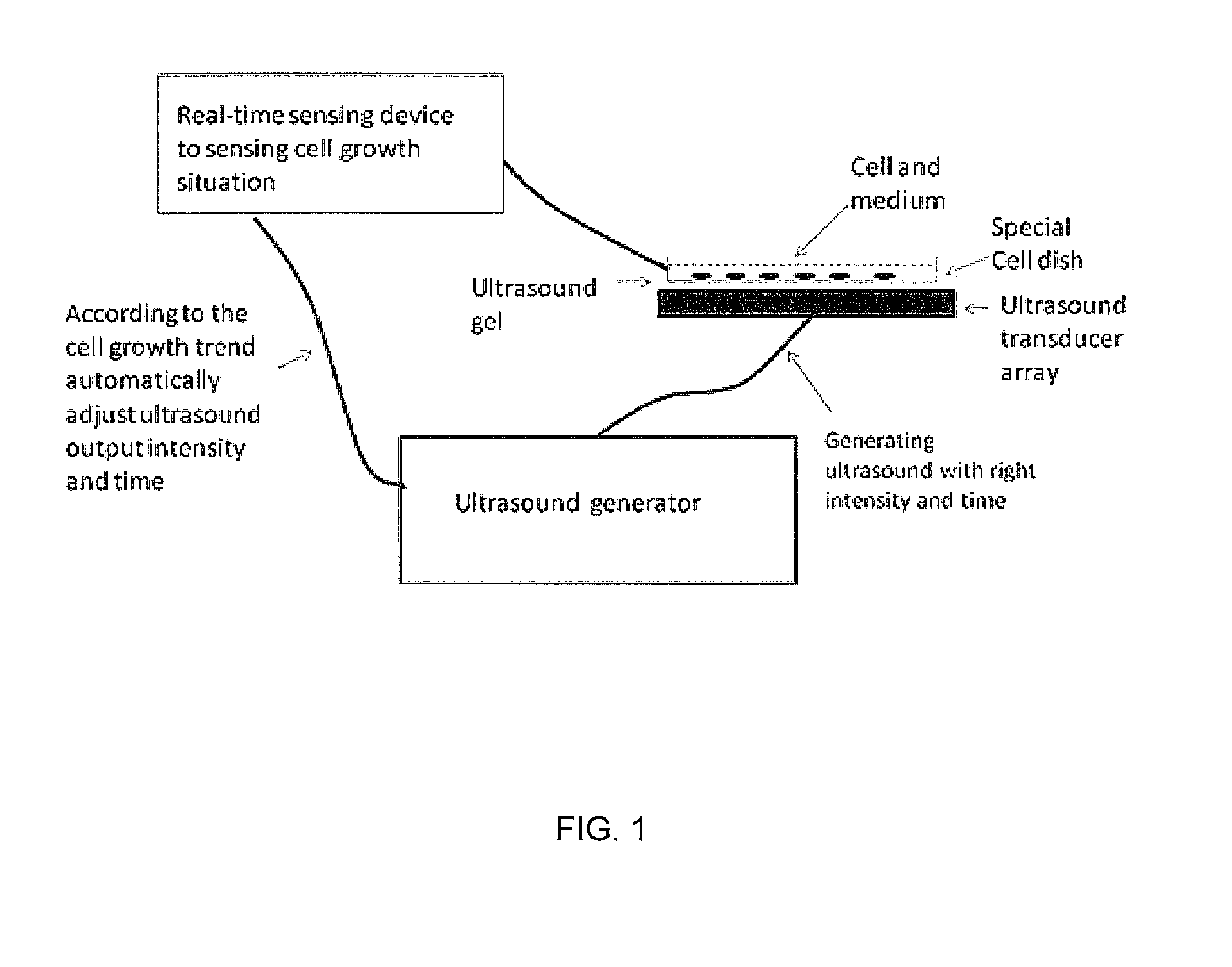
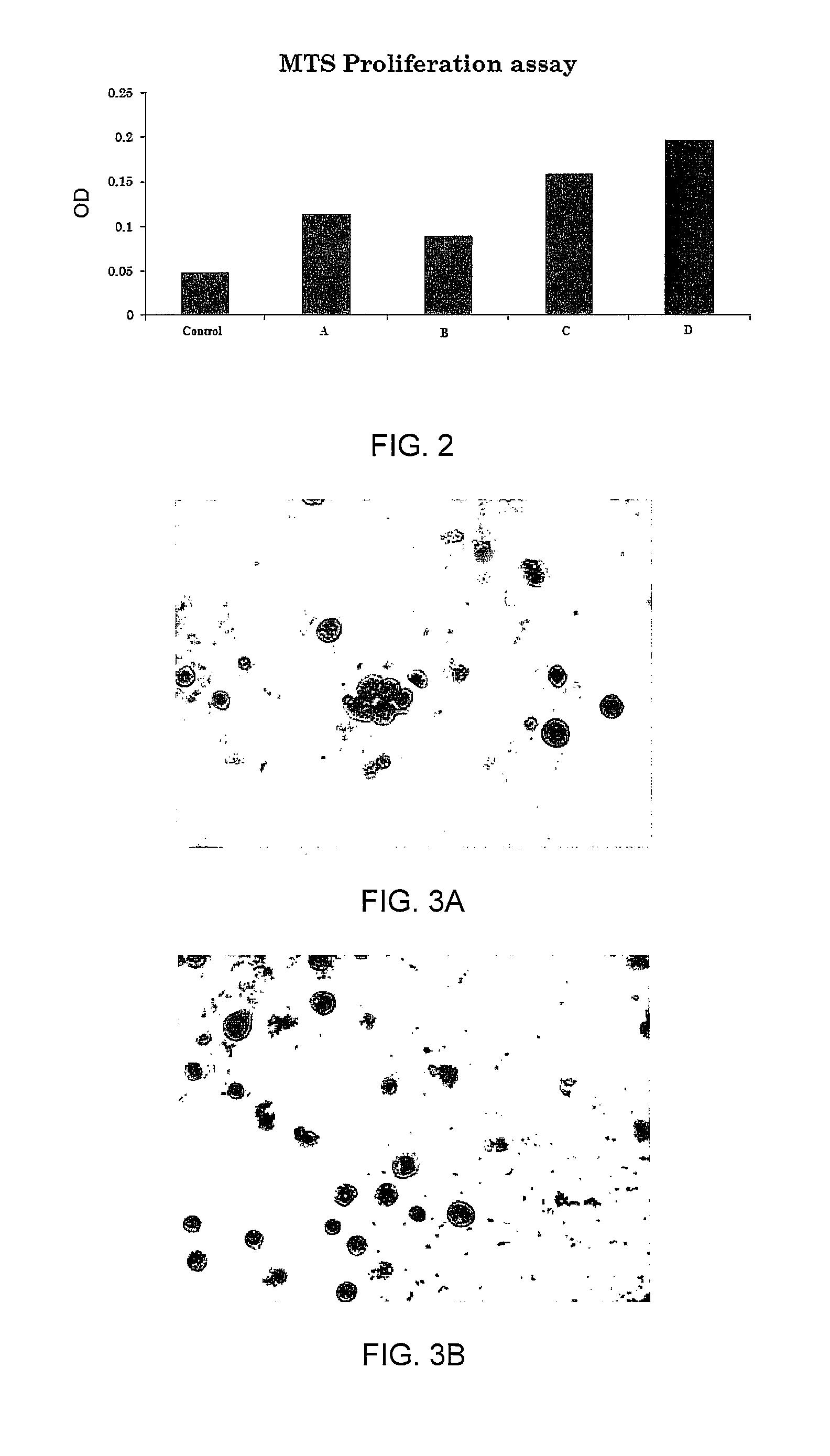
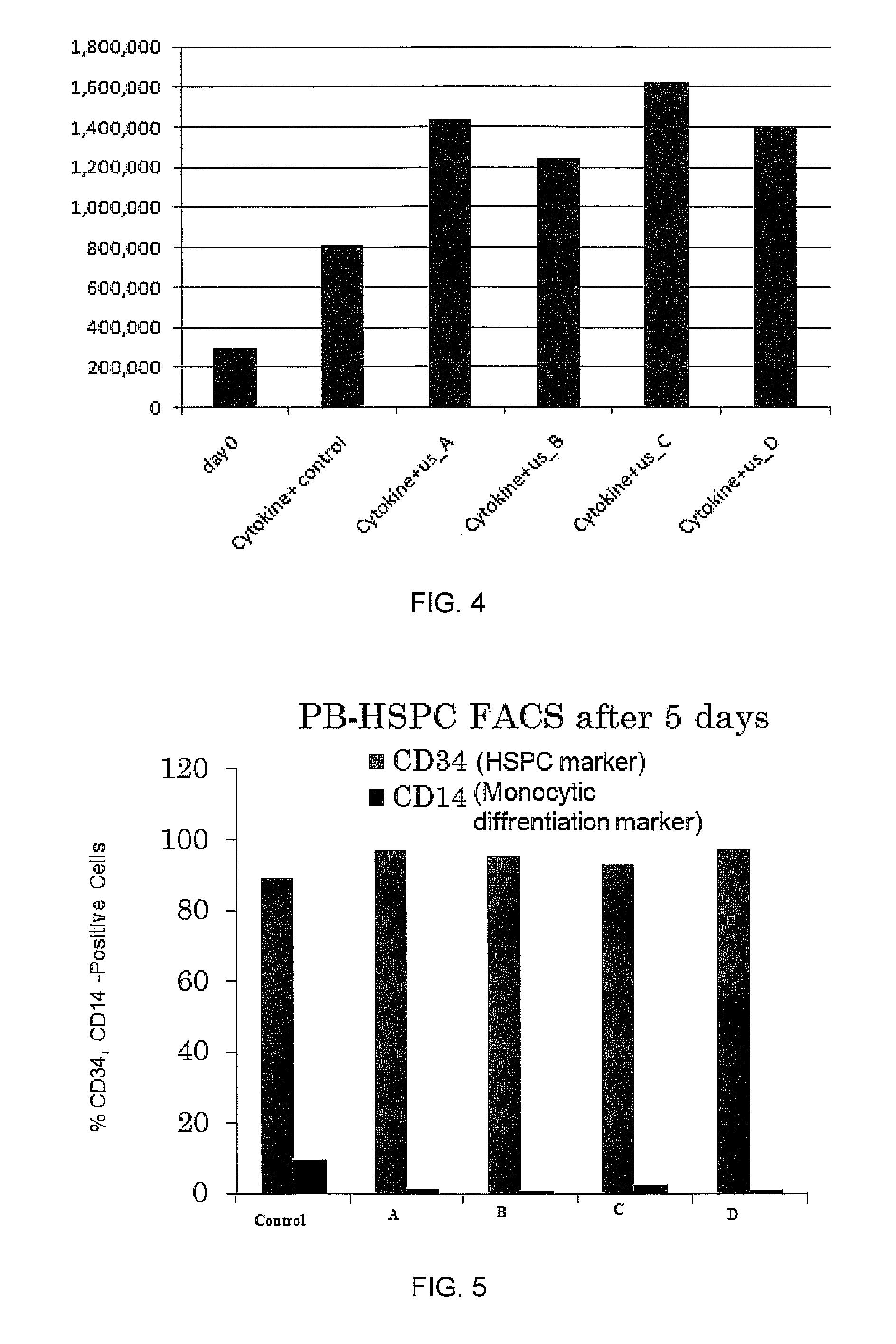
Enhanced animal cell growth using ultrasound
ActiveUS20130022957A1Enhancing of monoclonal antibody productionMinimize temperature increaseMicrobiological testing/measurementCulture processCell growthBiology
A method of increasing animal cell growth and monoclonal antibody production in an animal cell or cell culture includes the use of ultrasound at a frequency greater than about 1 MHz.
Owner:HIDACA
Preparation method of novel coronavirus nucleocapsid protein monoclonal antibody
InactiveCN112111007AAvoid the risk of only recognizing polypeptide antigens but not N proteinQuick identificationSsRNA viruses positive-senseVirus peptidesAntigen epitopeAntigen
The invention discloses a preparation method of a novel coronavirus nucleocapsid protein monoclonal antibody. The preparation method comprises the following steps: S1, synthesizing polypeptides; S2, preparing an immunogen; S3, preparing a screening antigen; S4, immunizing animals; S5, fusing cells; S6, screening and detecting; S7, establishing a stable cell strain; S8, producing the monoclonal antibody; S9, coupling the antibody; and S10, screening paired antibodies. The polypeptides are synthesized by selecting different antigen epitopes at two ends of N protein respectively and are used as antigens; the prepared monoclonal antibody can realize direct pairing; in a polypeptide synthesis process, a connecting bridge is added and steric hindrance is reduced, so that the antigen epitopes aresufficiently exposed on the surface of carrier protein and the antigens can be rapidly identified; recombinant protein is selected as a detection antigen, so that the risk that the screened antibodyonly identifies polypeptide antigens and does not identify the N protein is avoided; and whether a sample contains novel coronaviruses or not can be directly detected by detecting the content of the Nprotein.
Owner:通用生物(安徽)股份有限公司
Method for preparing monoclonal antibody of spike protein of novel coronavirus
The invention discloses a method for preparing a monoclonal antibody of a spike protein of novel coronavirus. The method comprises the following steps: antigen preparing, animal immunizing, cell fusing, screening detecting, stable cell line establishing and monoclonal antibody producing. The spike protein is a superficial membrane protein of SARS-CoV-2 and contains two subunits S1 and S2, the S1 mainly comprises one receptor binding domain and is responsible for identifying a cell surface receptor, and the S2 comprises fundamental elements required for membrane fusion. The S protein reacts with an antibody and T cells in induction and plays a crucial role in protective immunization, so that the monoclonal antibody of the spike protein can be excellently bound to the spike protein to form acomplex, the complex can be digested or degraded, then, the SARS-CoV-2 virus can be deactivated, and the effect of killing the SARS-CoV-2 virus is achieved.
Owner:通用生物(安徽)股份有限公司
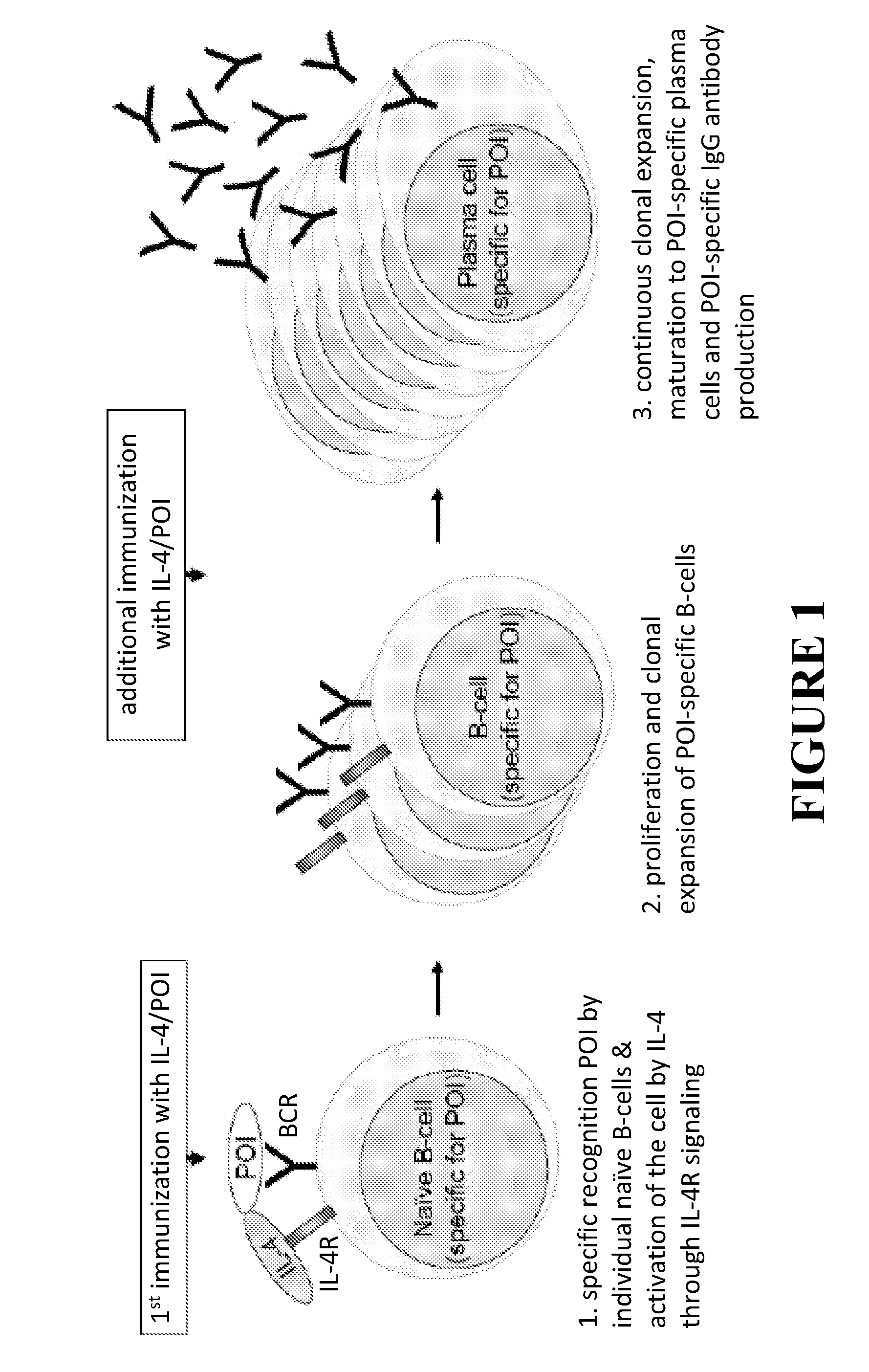
Methods for monoclonal antibody production
InactiveUS20110287454A1Simple methodImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsPresent methodTh2 cytokines
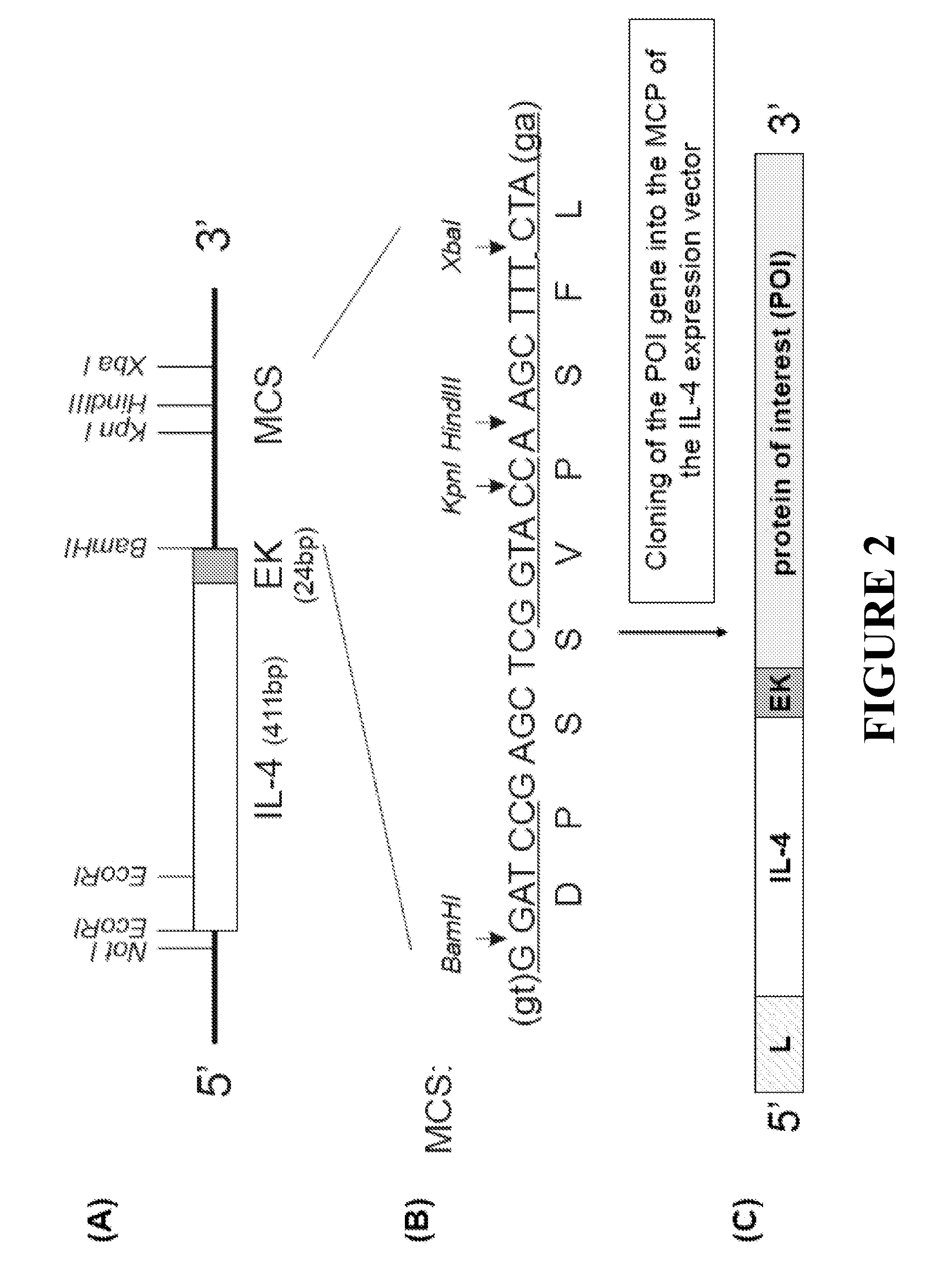
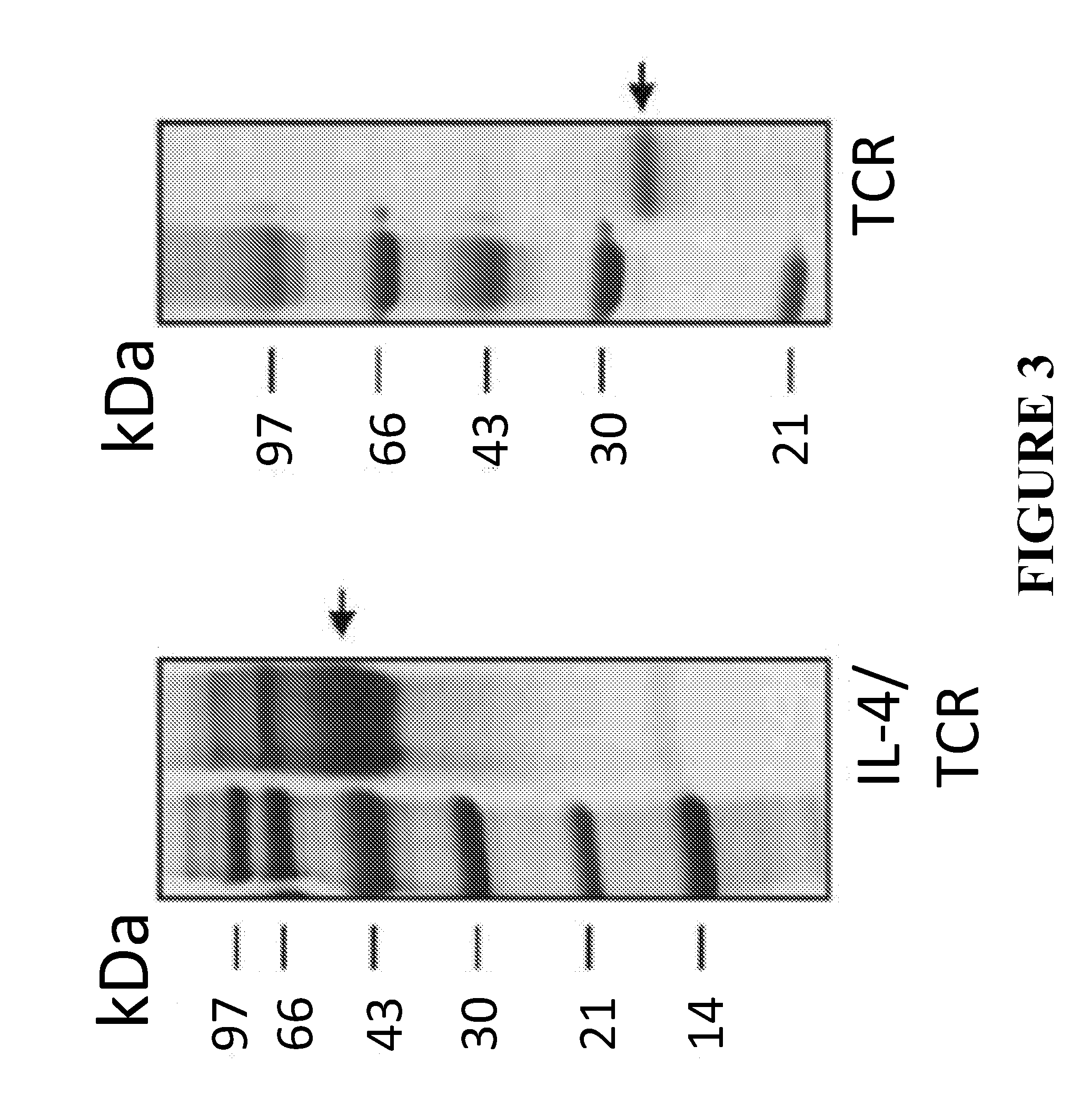
This invention provides improved methods for production of monoclonal antibodies against a protein of interest. The present methods are based on immunization of an animal with a fusion protein between a protein of interest and a Th2 cytokine such as IL-4, IL-5, IL-13 and IL-31.
Owner:CORNELL UNIVERSITY
Method for producing monoclonal antibody by using hybridoma and produced monoclonal antibody
PendingCN109265539AConsistent concentrationConsistent volumeImmunoglobulinsSerum freeCell culture media
The invention discloses a method for producing a monoclonal antibody by using hybridoma. The method comprises the following steps: placing resuscitated hybridoma in a first cell culture medium for performing sub-culture and enlarge culture, wherein the volume fraction of serum in the first cell culture medium is larger than 10 percent; transferring the hybridoma into a second cell culture medium for performing enlarge culture, wherein the volume fraction of serum in the second cell culture medium is less than or equal to 10 percent; transferring the hybridoma into a serum-free cell culture medium for culturing, and performing monoclonal antibody production. By adopting the method, the first cell culture medium is used for performing eutrophic culture, and the second cell culture medium isused for performing uniform group culture, so that the concentration and volume of hybridoma of different batches can keep consistent at the start of antibody production, the whole production flow canbe widely applied to different hybridoma plants, and the growth period of the antibody is stable. The monoclonal antibody produced in the serum-free cell culture medium has high product quality.
Owner:ZHEJIANG ZHENGXI BIOMEDICAL CO LTD
Anti-human-liver-cancer-stem-cell monoclonal antibody
ActiveCN106366190AGrowth inhibitionPrevent recurrenceImmunoglobulins against animals/humansMicroorganism based processesIn vivoDrug resistance
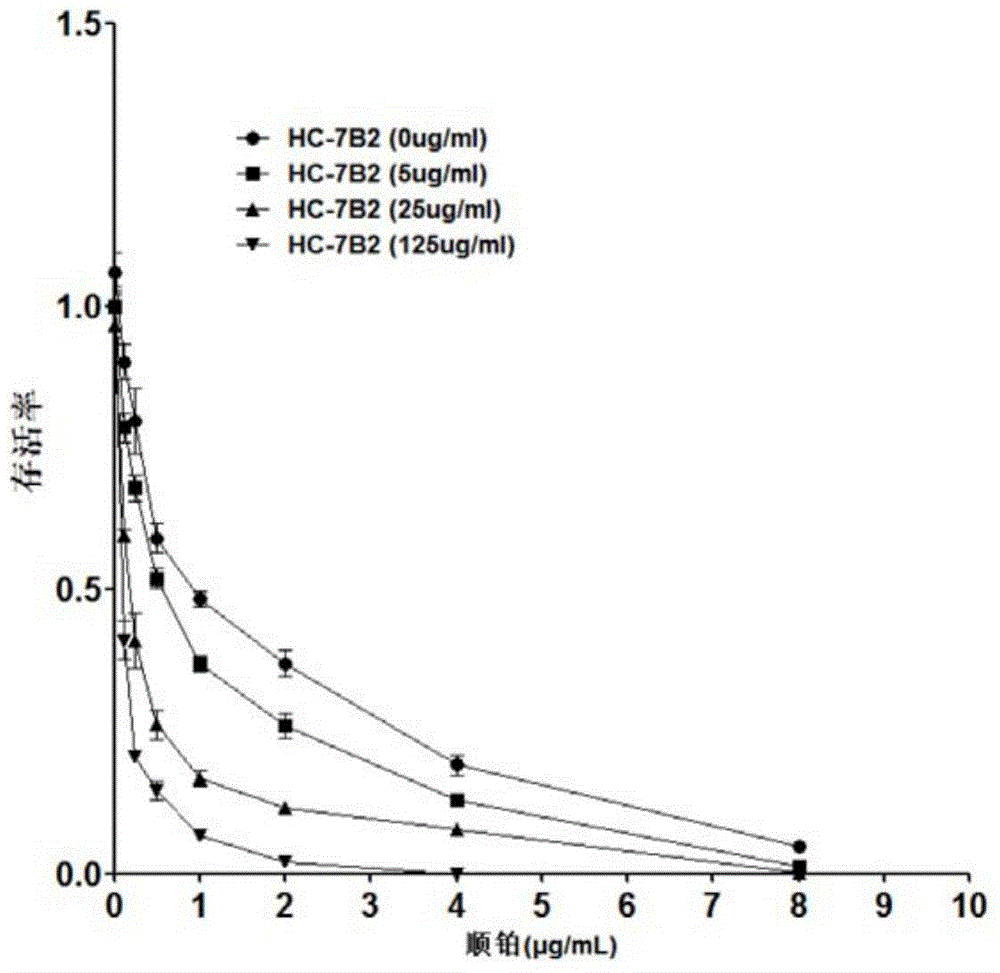
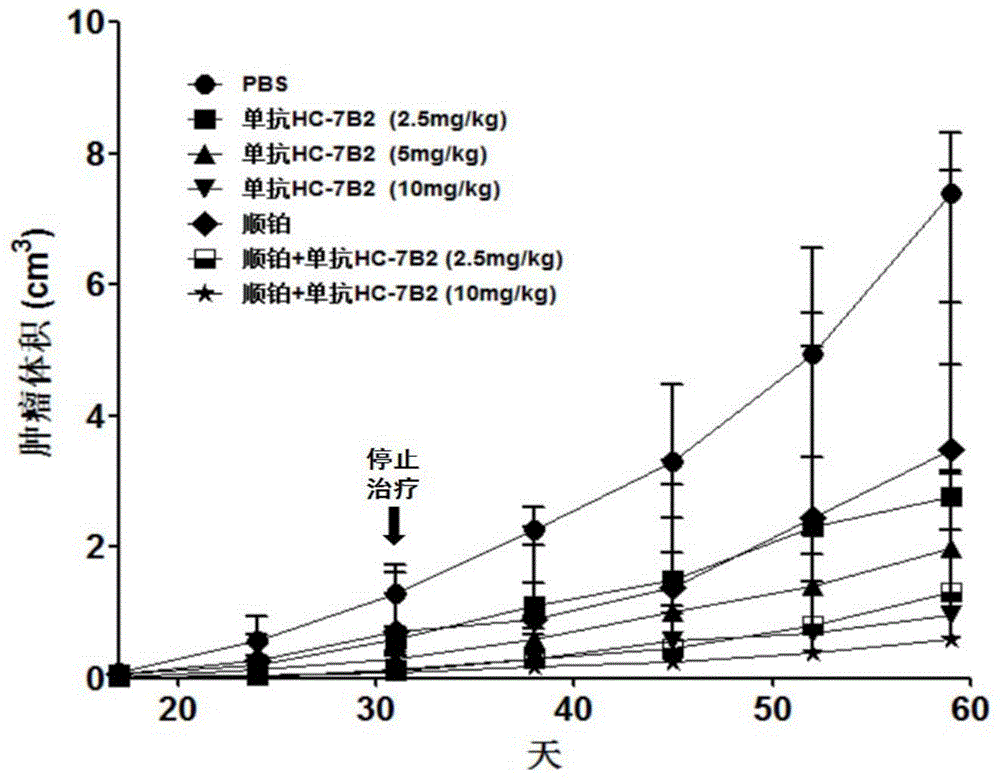
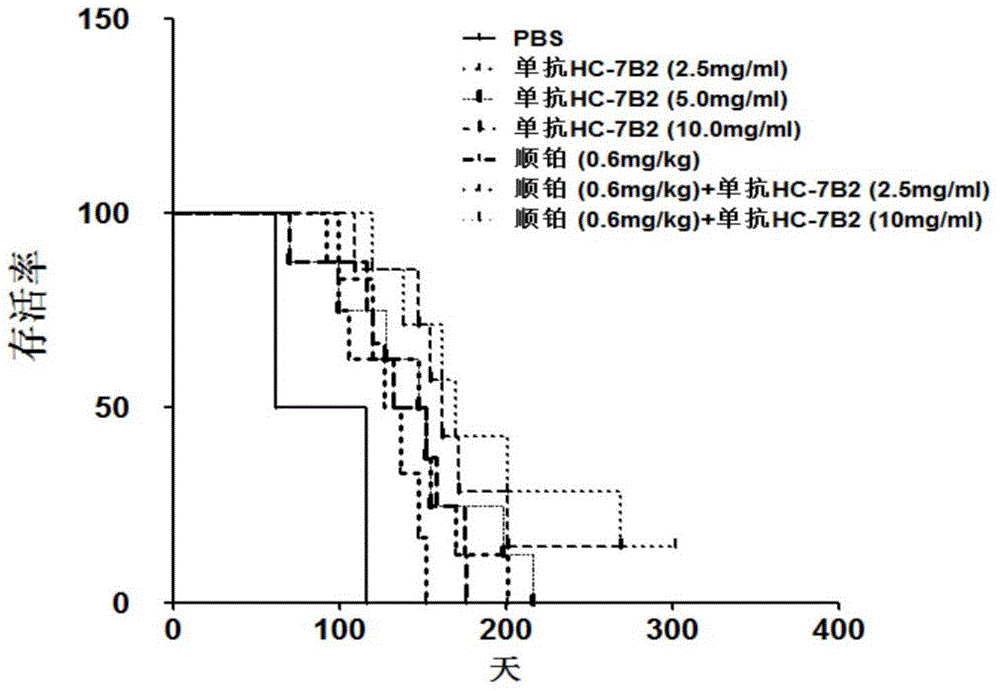
The present invention discloses an anti-human-liver-cancer-stem-cell monoclonal antibody, and particularly relates to a new monoclonal antibody capable of inhibiting human liver cancer stem cells in a targeting manner, a hybridoma cell line secreting the monoclonal antibody, and applications of the monoclonal antibody in preparation of drugs for treatment of human liver cancer recurrence and drug resistance. According to the present invention, the monoclonal antibody can significantly inhibit the self-renewal, the invasion and the drug resistance of human liver cancer stem cells in vitro, can significantly inhibit the growth of human liver cancer transplantation tumors in vivo, and prolongs the survival time of tumor-bearing mice. The present invention further provides a monoclonal antibody production cell line mouse hybridoma cell line HC-7B2 (with the preservation number of CGMCC No.10897).
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Monoclonal antibody production by EBV transformation of B cells
ActiveUS8071371B2Improve efficiencyRapid isolationMicrobiological testing/measurementGenetically modified cellsEpstein bar virusPolyclonal B cell activator
A method for producing a clone of an immortalized human B memory lymphocyte, comprising the step of transforming human B memory lymphocytes using Epstein Barr Virus (EBV) in the presence of a polyclonal B cell activator. The method is particularly useful in a method for producing a clone of an immortalized human B memory lymphocyte capable of producing a human monoclonal antibody with a desired antigen specificity, comprising the steps of: (i) selecting and isolating a human memory B lymphocyte subpopulation; (ii) transforming the subpopulation with Epstein Barr Virus (EBV) in the presence of a polyclonal B cell activator; (iii) screening the culture supernatant for antigen specificity; and (iv) isolating an immortalized human B memory lymphocyte clone capable of producing a human monoclonal antibody having the desired antigen specificity.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE
Feeding strategies and purification processes for monoclonal antibody production
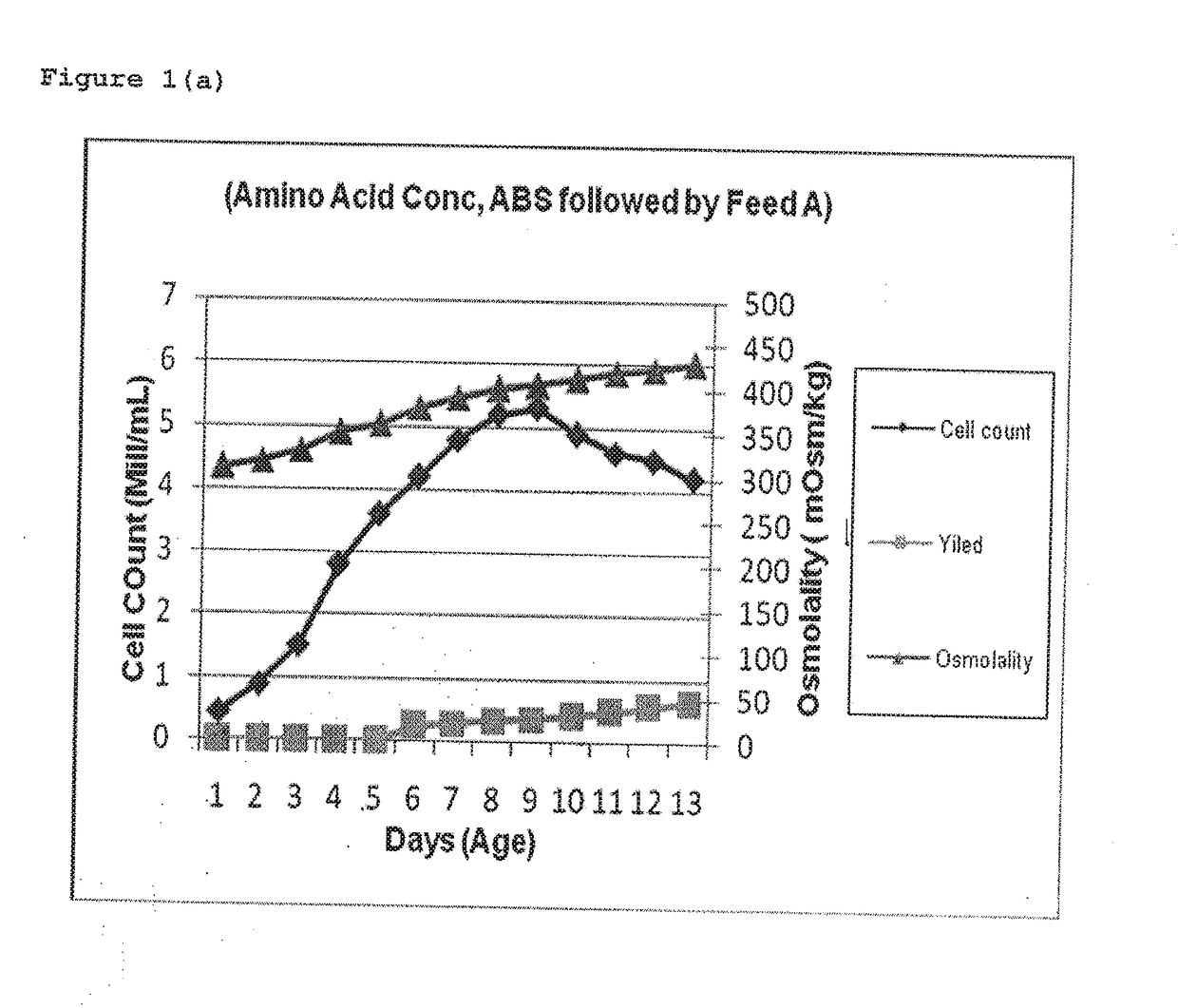
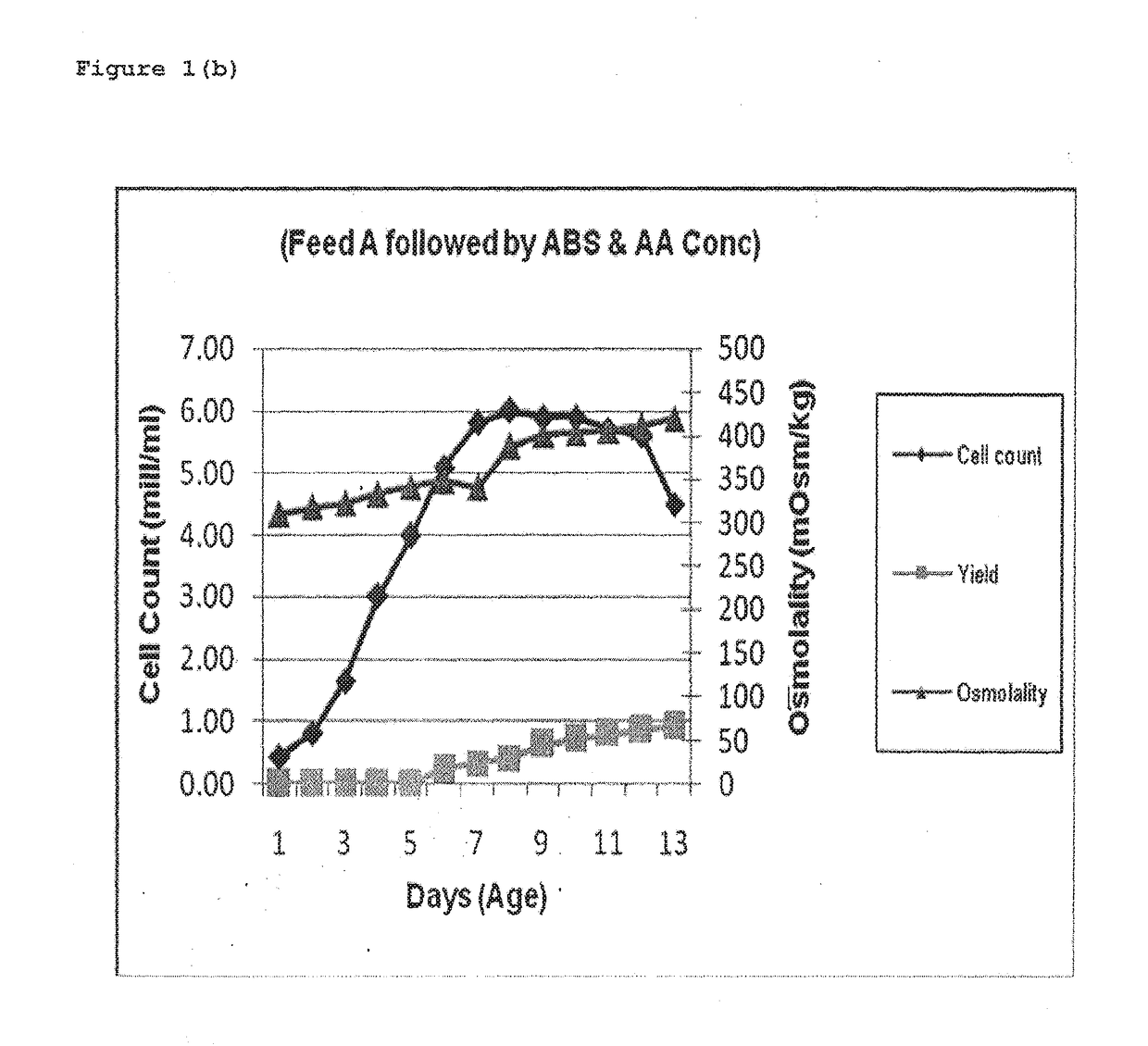
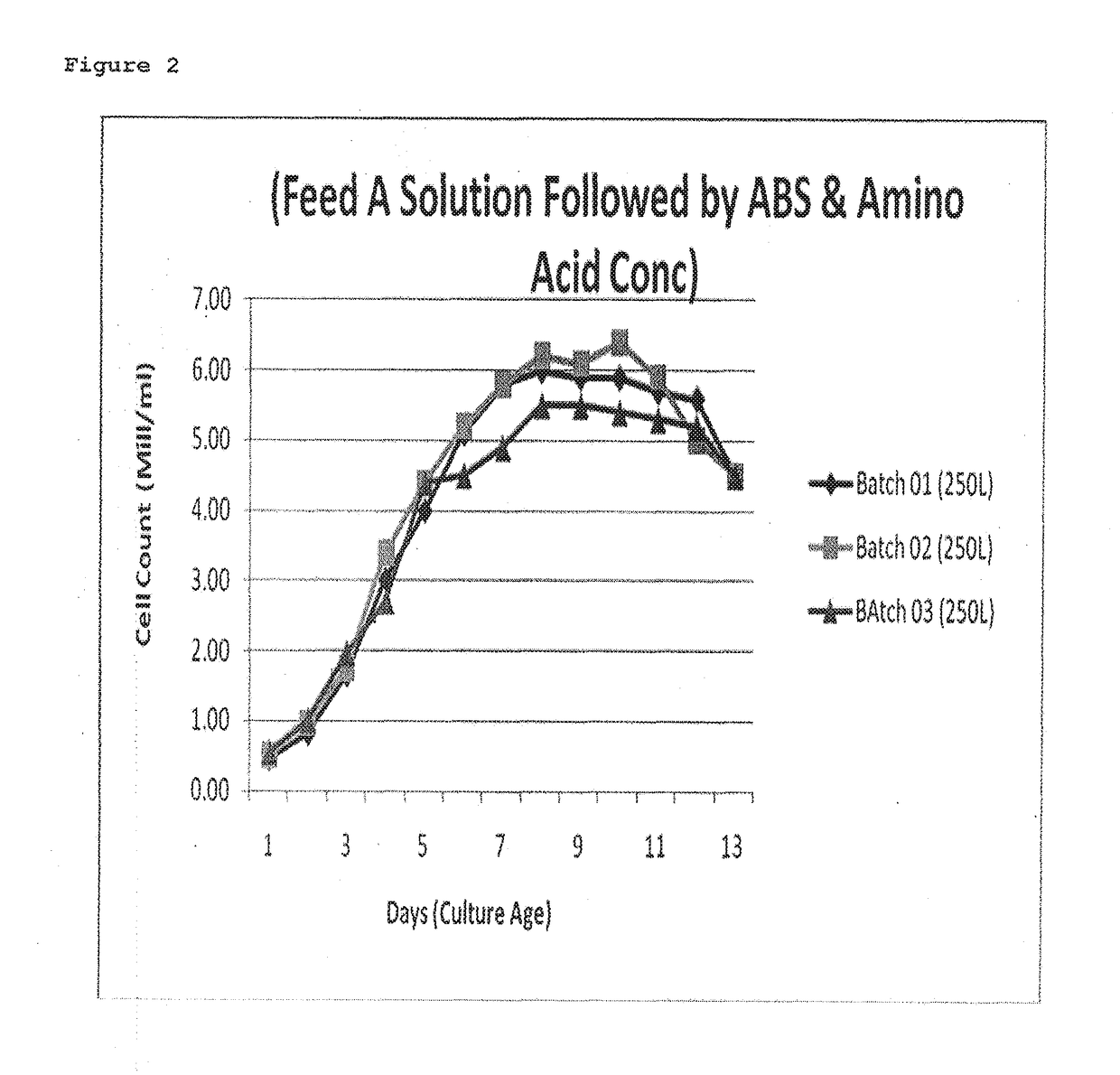
ActiveUS20170088873A1Quantity minimizationIncrease productionImmunoglobulins against virusesPeptide preparation methodsMetaboliteRabies
This invention provides an improved process for manufacturing a Rabies monoclonal antibody (HuMab 17C7) that results in low osmolality, minimum secondary metabolites like ammonia and lactate, enhanced cell growth and productivity, minimum aggregation or degradation of monoclonal antibody during purification, thereby improving potency of monoclonal antibody (HuMab 17C7) as compared to human rabies immunoglobulin (hRIG).
Owner:SERUM INST OF INDIA PTE LTD
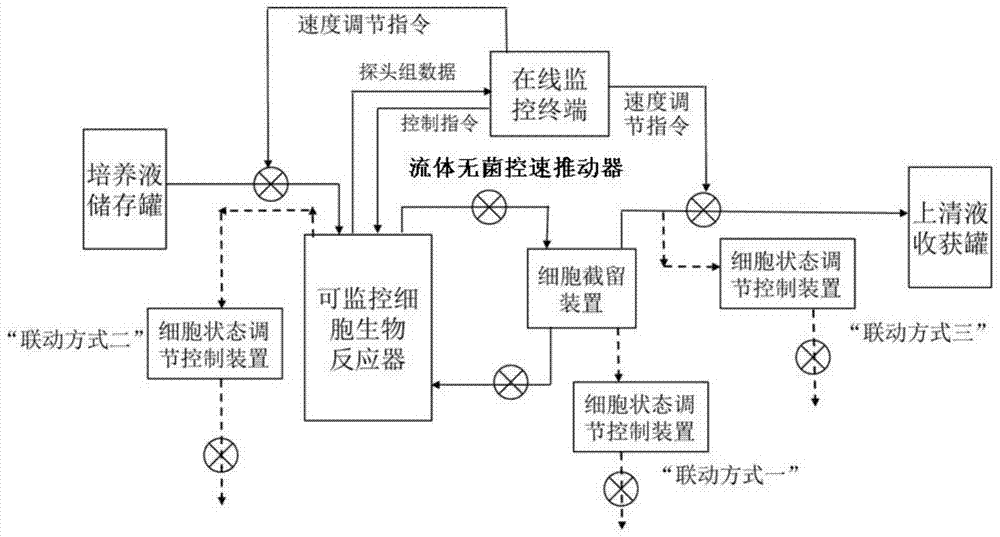
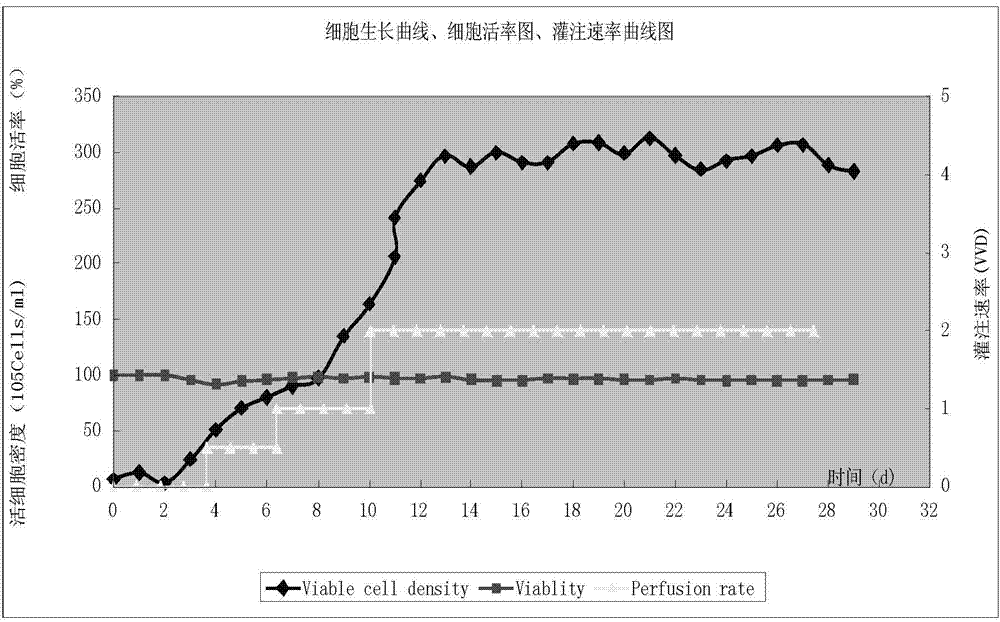
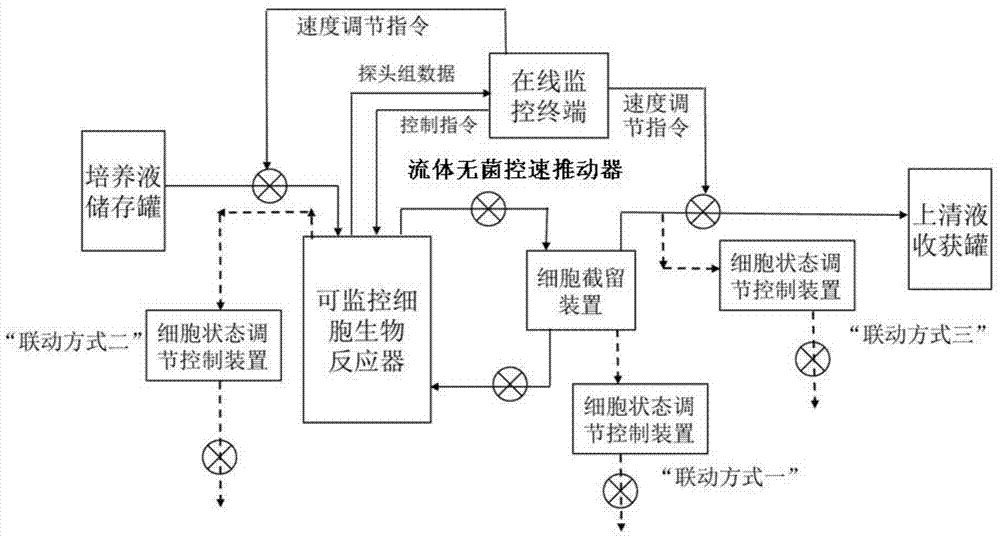
Apparatus and method for producing anti-CD52 monoclonal antibodies through online industrially robust regulation of cell states
InactiveCN104845881AStable growth environmentConstant vigorBioreactor/fermenter combinationsBiological substance pretreatmentsControl cellCvd risk
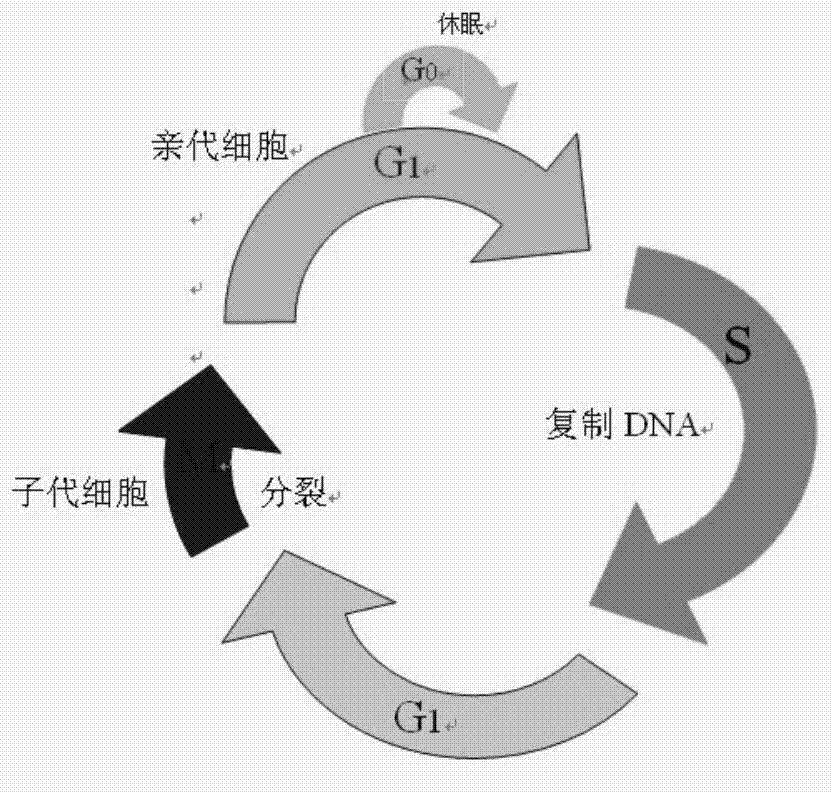
The present invention discloses a bioreactor cell culture apparatus capable of onlinely and continuously monitoring physiological and biochemical indexes in a variety of cell culture environments and directly regulating and controlling cell states (including cell cycle and the like), and a method for producing anti-CD52 monoclonal antibodies through the apparatus. With the apparatus and the method of the present invention, the bacterial pollution risk during the cell culture process can be reduced, the stable cell growth environment can be maintained, the constant cell growth viability can be maintained, and the high-quality, high-efficiency and continuous anti-CD52 monoclonal antibody production can be achieved.
Owner:上海泰因生物技术有限公司
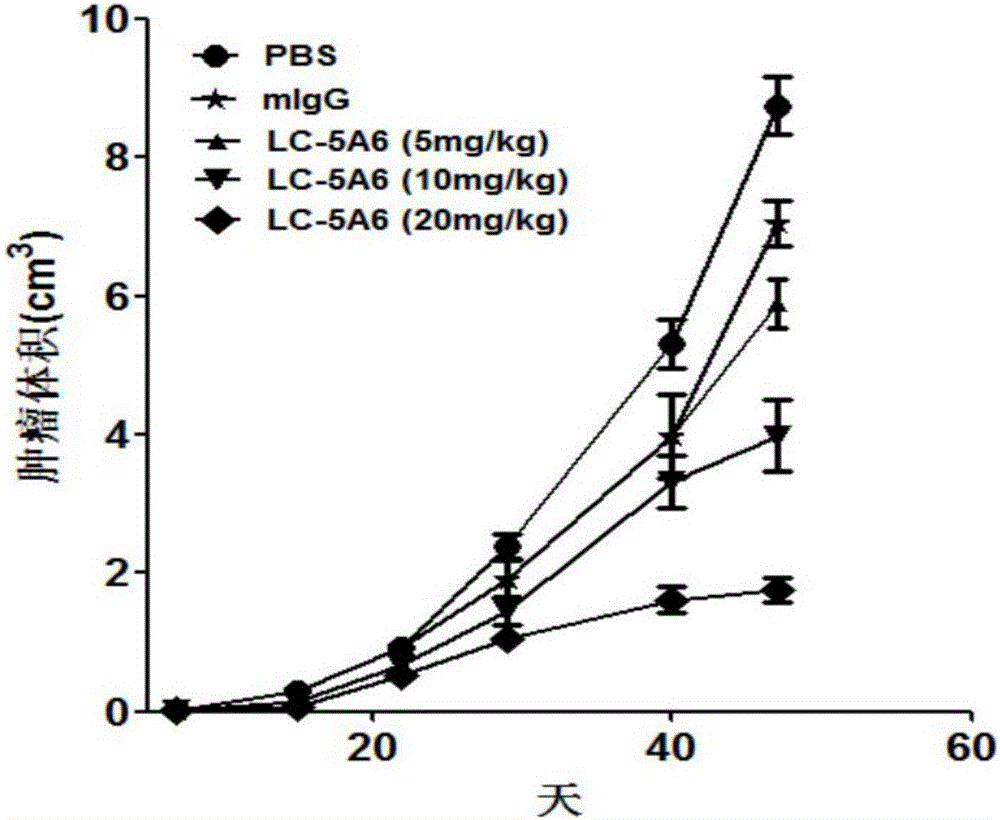
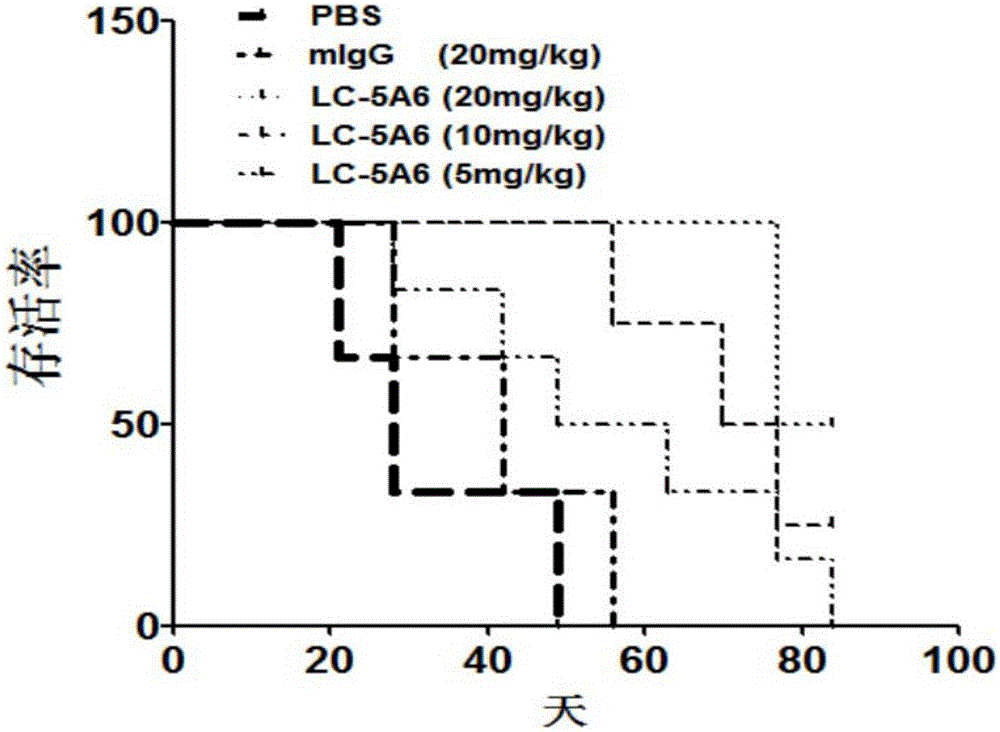
Anti-human-lung-cancer-stem-cell monoclonal antibody
InactiveCN106366189AInhibition of self-renewalInhibit migrationImmunoglobulins against animals/humansMicroorganism based processesIn vivoHybridoma cell
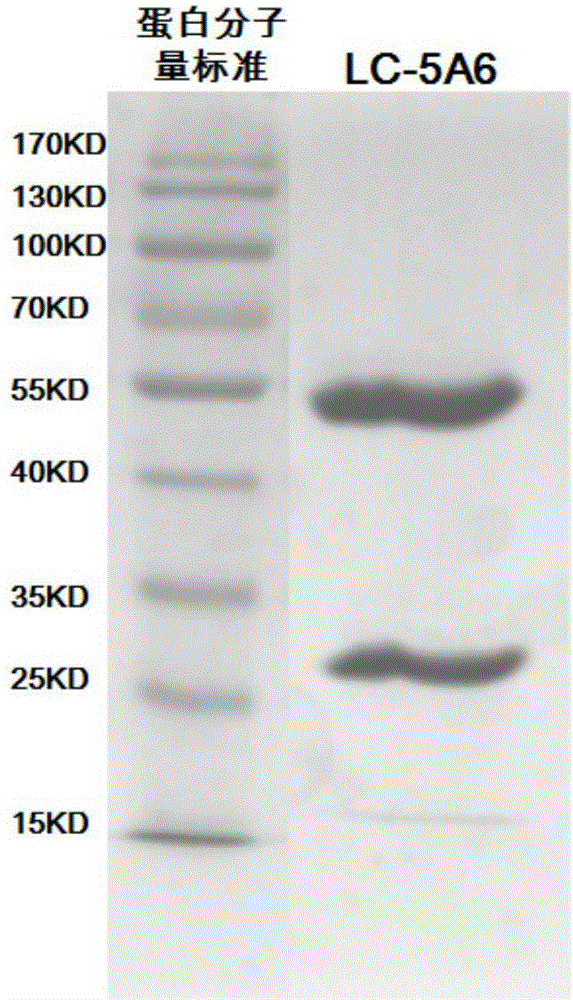
The present invention discloses an anti-human-lung-cancer-stem-cell monoclonal antibody, and particularly relates to a new monoclonal antibody capable of specifically recognizing and inhibiting human lung cancer stem cells, a hybridoma cell line for producing the monoclonal antibody, and applications of the monoclonal antibody in preparation of drugs for treatment of human lung cancer recurrence. According to the present invention, the monoclonal antibody can significantly inhibit the self-renewal, the invasion and the migration of human lung cancer stem cells in vitro, can significantly inhibit the growth and the recurrence of human lung cancer transplantation tumors in vivo, and prolongs the survival time of tumor-bearing mice. The present invention further provides a monoclonal antibody production cell line mouse hybridoma cell line LC-5A6 having the preservation number of CGMCC No.10898.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Preparation method of alicyclic acid bacillus resisting monoclonal antibody
InactiveCN104650223AFor long-term storageLow costImmunoglobulins against bacteriaSpleen cellAbdominal cavity
The invention relates to a preparation method of an alicyclic acid bacillus resisting monoclonal antibody. The method comprises the following steps: (1) preparing an immunized mouse; (2) culturing myeloma cells for 3-4 days to obtain cultured mouse myeloma cells; (3) inoculating alicyclic acid bacillus in the abdominal cavity of the immunized mouse for immunization and obtaining spleen cells; dispersing the spleen cells to obtain a spleen cell precipitate; (4) centrifuging the cultured mouse myeloma cells to obtain a myeloma cell precipitate; (5) respectively suspending and mixing the spleen cell precipitate and the myeloma cell precipitate in a DMEM high-sugar culture solution, and centrifuging to obtain a precipitate; centrifuging and suspending the precipitate to obtain fused cells; (7) culturing and assaying the fused cells to determine hybridoma cells; cloning and screening the hybridoma cells to obtain a hybridoma cell line; and (8) preparing a monoclonal antibody, namely performing monoclonal antibody production on the hybridoma cell line according to a general method. The preparation method disclosed by the invention is rapid and high in specificity.
Owner:兰州海关技术中心
Preparation method of regenerated gene protein REG1 alpha monoclonal antibody
PendingCN111909266AGuaranteed specificityRapid detection and screeningImmunoglobulins against animals/humansBiological material analysisGenes proteinsMyeloid Tumor
The present invention relates to a preparation method of a regenerated gene protein REG1 alpha monoclonal antibody. The method comprises the following steps: (1) preparing immunized mice; (2) culturing myeloma cells for 3-4 days to obtain cultured mouse myeloma cells; (3) dissolving REG1 alpha protein, inoculating abdominal cavity of the immunized mice with the dissolved REG1 alpha protein for immunization, obtaining splenocytes, and dispersing the splenocytes to obtain a splenocyte precipitate; (4) centrifuging the cultured mouse myeloma cells to obtain a myeloma cell precipitate; (5) respectively suspending the splenocyte precipitate and the myeloma cell precipitate by using a DMEM high-glucose culture solution, conducting mixing, and centrifuging the mixture to obtain a precipitate; (6)centrifuging and suspending the precipitate to obtain fusion cells; (7) culturing and determining the fusion cells, and determining hybridoma cells; (8) screening and separating monoclonal antibodies; and (9) preparing a large amount of monoclonal antibodies, namely producing the monoclonal antibodies from a hybridoma cell line according to a conventional method. The preparation method is rapid and high in specificity.
Owner:甘肃智选生物科技有限公司
Enhanced animal cell growth using ultrasound
ActiveUS8962290B2Enhancing rate of cell growth and monoclonal antibody productionMinimize temperature increaseMicrobiological testing/measurementCulture processCell growthBiology
A method of increasing animal cell growth and monoclonal antibody production in an animal cell or cell culture includes the use of ultrasound at a frequency greater than about 1 MHz.
Owner:HIDACA
Affinity purification method for lowering protein content of host cell in monoclonal antibody production
InactiveCN113621063AAffinity craft is simple and easyHigh yieldImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsComplementarity determining regionHeavy chain
The invention discloses an affinity purification method for lowering protein content of a host cell in monoclonal antibody production. The method comprises the following steps: balancing an affinity chromatography medium by using a first balance buffer solution to obtain a balanced affinity chromatography medium, and combining monoclonal antibody fermentation liquor with the balanced affinity chromatography medium; and then, carrying out intermediate pre-elution leaching, and then, carrying out final elution to remove host-cell proteins so as to obtain a monoclonal antibody. The monoclonal antibody is a separated monoclonal antibody against a human interferon alpha receptor 1 (IFNAR1), and comprises three heavy-chain complementary determining regions (CDR-H1, CDR-H2 and CDR-H3) and three light-chain complementary determining regions (CDR-L1, CDR-L2 and CDR-L3). The method is simple and easy to implement, amplified purification production can be carried out, cell fermentation supernatant does not need pretreatment, the elution sample yield is relatively high, meanwhile, a HCP residual quantity is also kept at a relatively low level (the residual control quantity is not higher than 0.1%), and therefore, the pressure for removing HCP in a subsequent purification step is relieved.
Owner:QYUNS THERAPEUTICS CO LTD +1
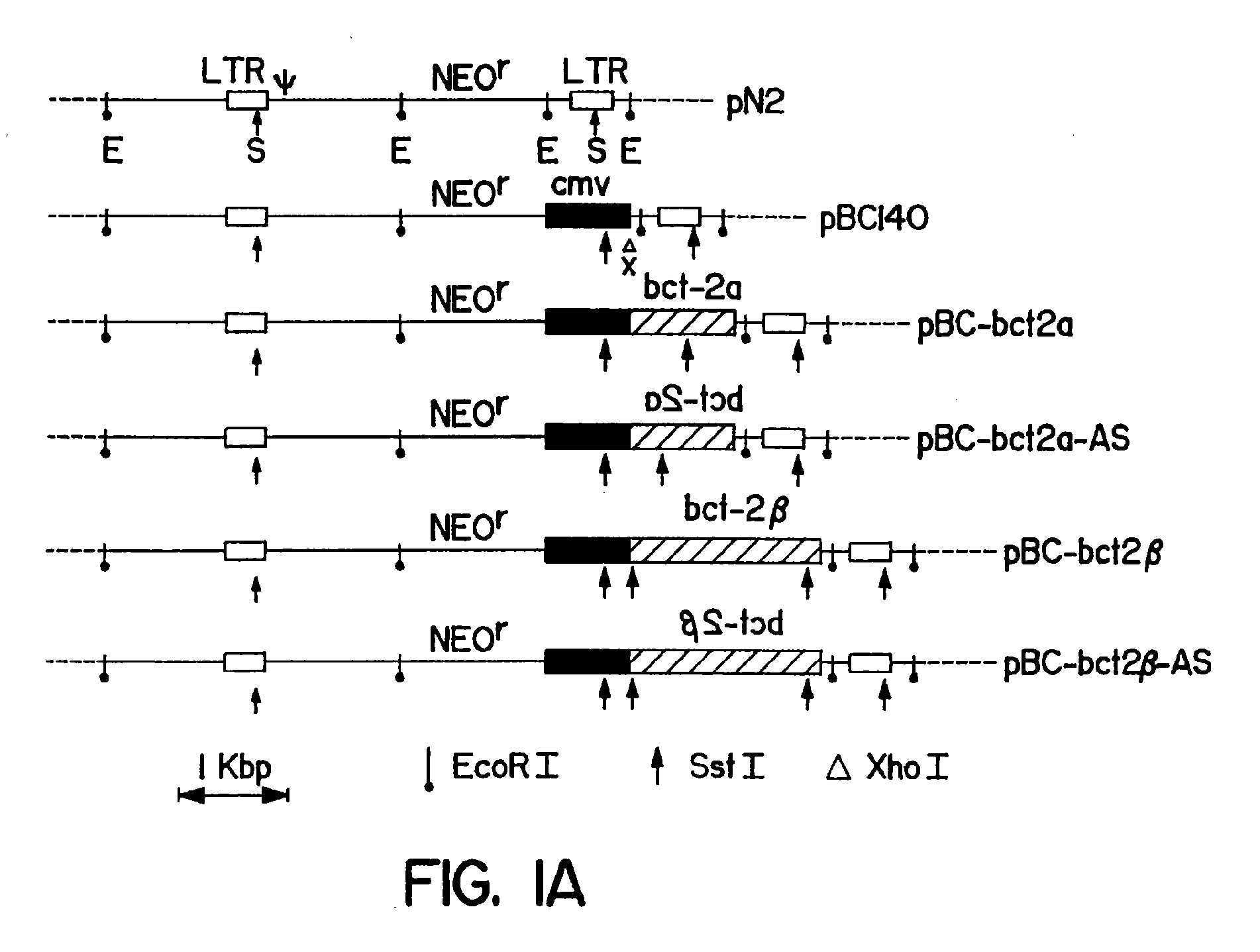
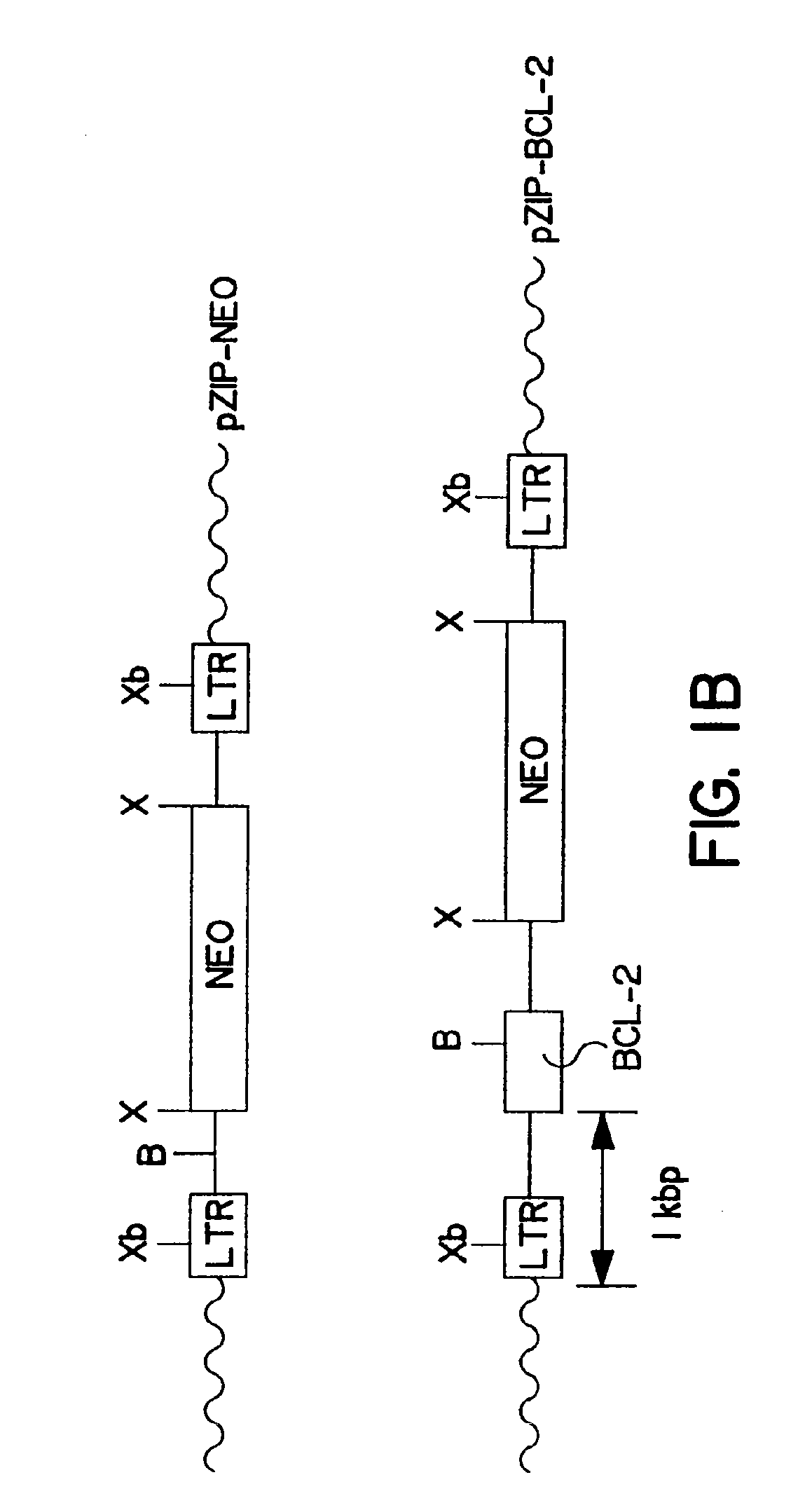
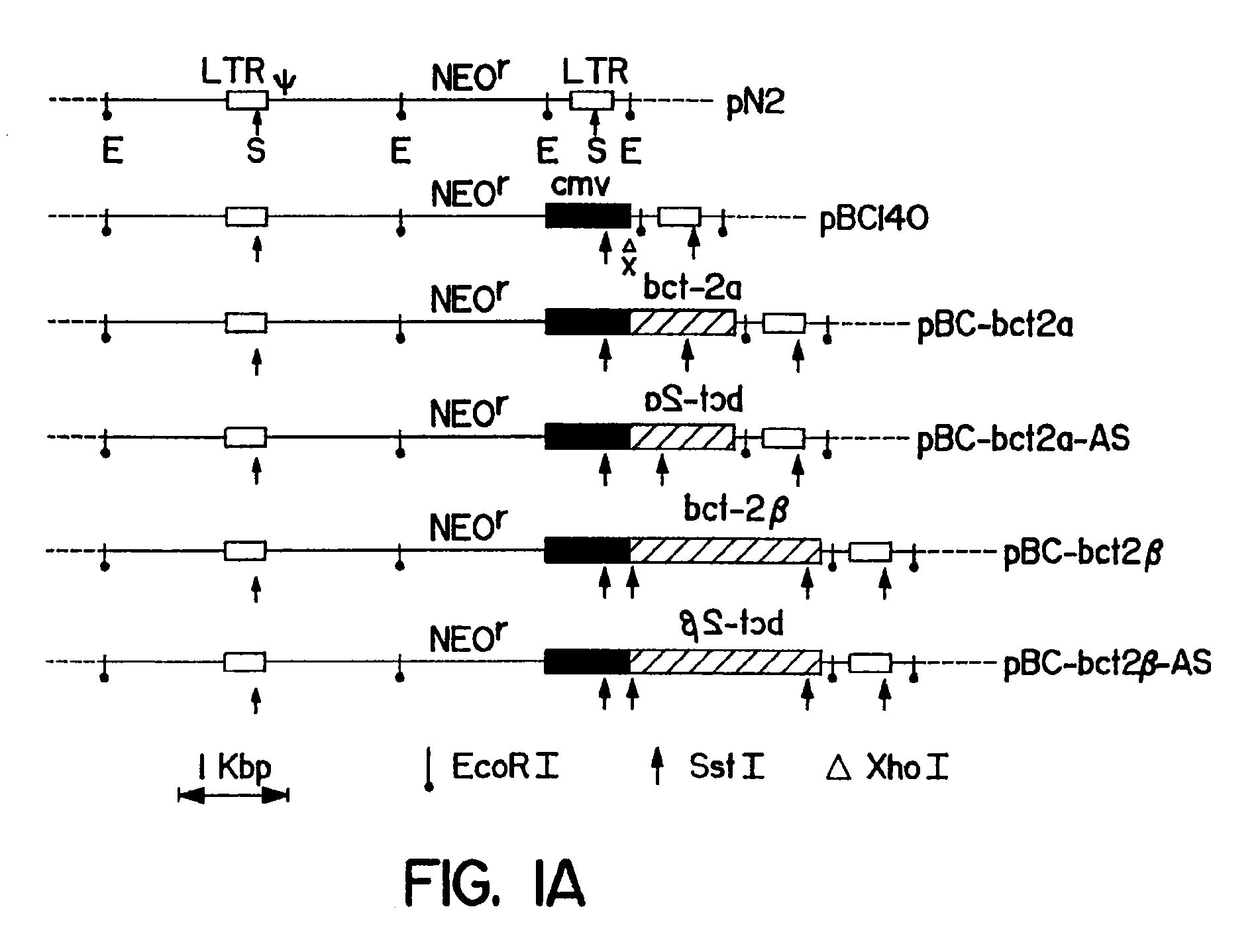
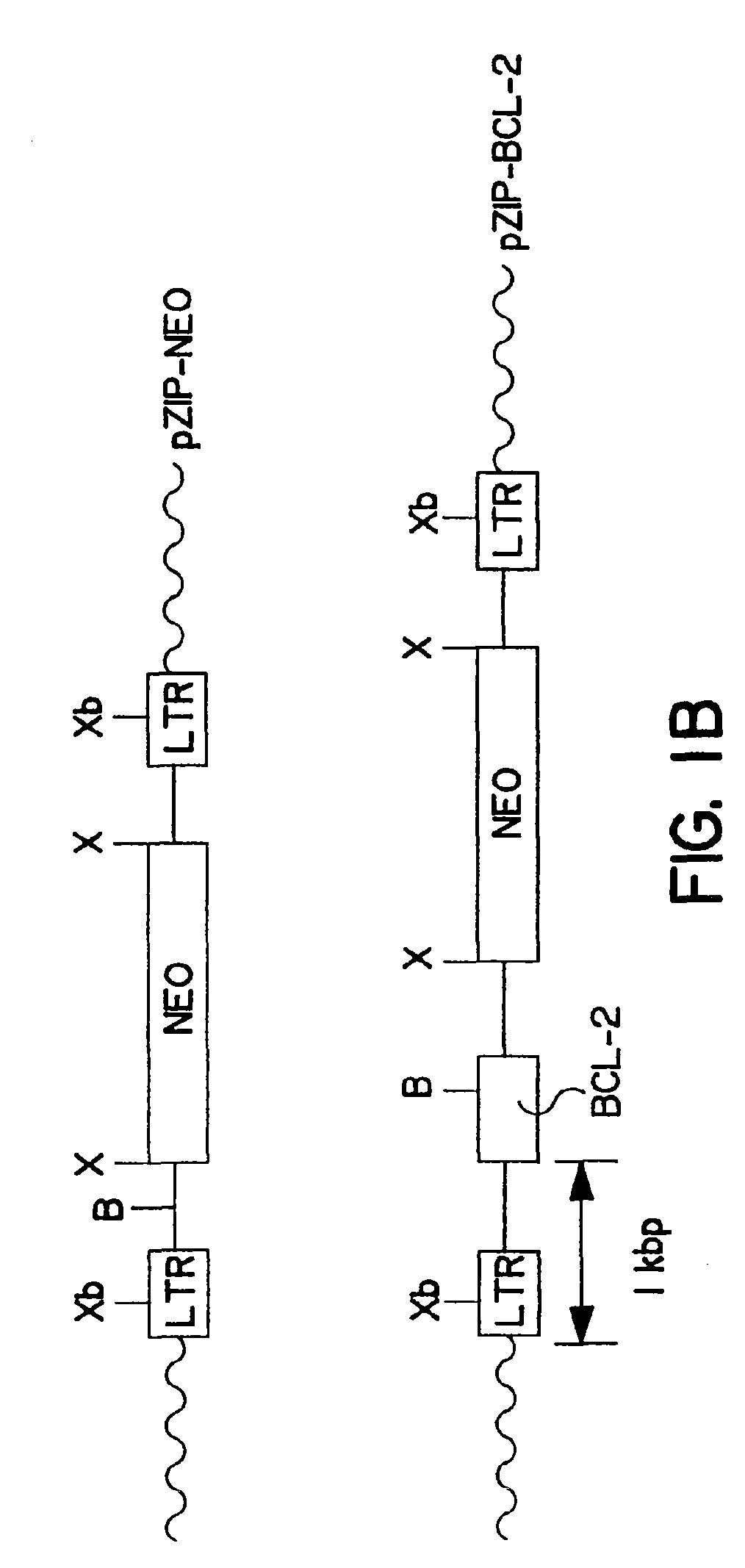
Methods of using bcl-2 for the therapeutic treatment and prevention of diseases
InactiveUS20080102060A1High activityProlonging in vivo survivalPeptide/protein ingredientsGenetic material ingredientsNeuro-degenerative diseaseIn vivo
The invention provides a method of treating a disease or pathological condition resulting in apoptotic cell death. The method includes increasing the activity of Bcl-2 in cells affected by the disease or pathological condition. Diseases or pathological conditions can include, for example, neurodegenerative diseases, cancer and viral infections. Also provided is a method of prolonging the in vivo survival of transplanted cells for the treatment of a disease or pathological condition. The method includes increasing the activity of Bcl-2 in a population of cells and transplanting the population of cells having increased Bcl-2 activity into a subject. Diseases or pathological conditions can include, for example, neurodegenerative diseases, cancer and viral infections. A method to enhance the sensitivity of malignant cells to therapy is provided that includes decreasing the activity of Bcl-2 in the malignant cells. Methods to identify compounds that alter apoptotic cell death and to enhance monoclonal antibody production are also provided by the invention disclosed herein.
Owner:SANFORD BURNHAM MEDICAL RES INST
Methods of using BCL-2 for the therapeutic treatment and prevention of diseases
InactiveUS7910370B2High activityProlonging in vivo survivalPeptide/protein ingredientsGenetic material ingredientsDiseaseTherapeutic intent
Owner:SANFORD BURNHAM MEDICAL RES INST
Affinity purification method for reducing content of host cell protein in monoclonal antibody production
ActiveCN114057878AAffinity craft is simple and easyHigh yieldImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsComplementarity determining regionHeavy chain
The invention discloses an affinity purification method for reducing the content of host cell protein in monoclonal antibody production, which comprises the following steps: balancing an affinity chromatography medium by using a first equilibrium buffer solution to obtain a balanced affinity chromatography medium, and combining monoclonal antibody fermentation liquor with the balanced affinity chromatography medium; and then carrying out intermediate pre-elution and elution, and then carrying out final elution to remove host cell protein so as to obtain a monoclonal antibody, wherein the monoclonal antibody is a separated monoclonal antibody against human interferon alpha receptor 1 (IFNAR1), and comprises three heavy chain complementary determining regions (CDR-H1, CDR-H2 and CDR-H3) and three light chain complementary determining regions (CDR-L1, CDR-L2 and CDR-L3). The method is simple and easy to implement, amplification purification production can be carried out, the cell fermentation supernate does not need to be subjected to pretreatment, the elution sample yield is high, meanwhile, the HCP residual quantity is kept at a low level (the residual control quantity is not higher than 0.1%), and therefore the pressure for removing HCP in the subsequent purification step is relieved.
Owner:QYUNS THERAPEUTICS CO LTD
Hybridoma cell line producing monoclonal antibody against human d-dimer, preparation method and application
The invention relates to a hybridoma cell line of a monoclonal antibody against human D-dimer, a preparation method and application thereof. In the present invention, the purified D-dimer is used as an immunogen to immunize animal Balb / c mice, and the monoclonal antibody capable of stably secreting anti-D-dimer is obtained by cloning and screening by ELISA method through hybridoma technology through multiple cell fusions hybridoma cell lines. Monoclonal antibody production by mouse ascites method. And use the prepared anti-D-dimer monoclonal antibody as capture antibody and detection antibody, use D-dimer as detection antigen, carry out antibody pairing test, obtain the antibody pairing that can detect natural D-dimer and use This established a double-antibody sandwich ELISA method for the detection of D-dimer. Hybridoma cell lines secrete corresponding monoclonal antibodies, which can be matched and detected in natural serum, and a double-antibody sandwich ELISA method for detecting D-dimer has been preliminarily established.
Owner:CHONGQING TANSHENG TECH
Apparatus and method for producing anti-Her-2 monoclonal antibodies through online industrially robust regulation of cell states
InactiveCN104845883AStable growth environmentConstant vigorBioreactor/fermenter combinationsBiological substance pretreatmentsControl cellCell growth
The present invention discloses a bioreactor cell culture apparatus capable of onlinely and continuously monitoring physiological and biochemical indexes in a variety of cell culture environments and directly regulating and controlling cell states (including cell cycle and the like), and a method for producing anti-Her-2 monoclonal antibodies through the apparatus. With the apparatus and the method of the present invention, the bacterial pollution risk during the cell culture process can be reduced, the stable cell growth environment can be maintained, the constant cell growth viability can be maintained, and the high-quality, high-efficiency and continuous anti-Her-2 monoclonal antibody production can be achieved.
Owner:上海泰因生物技术有限公司
Anti-human nkx3.1 monoclonal antibody and its preparation method and application
ActiveCN113527481BImprove immune hit rateAntibody is stableGenetically modified cellsImmunoglobulins against animals/humansHumaninGenetic engineering
Owner:河南赛诺特生物技术有限公司
Unicellular fusion method for pig and mouse cells
PendingCN108707600AImprove fusion efficiencyImprove fusion cloning efficiencyHybrid cell preparationFermentationPolyethylene glycolAgglutinin
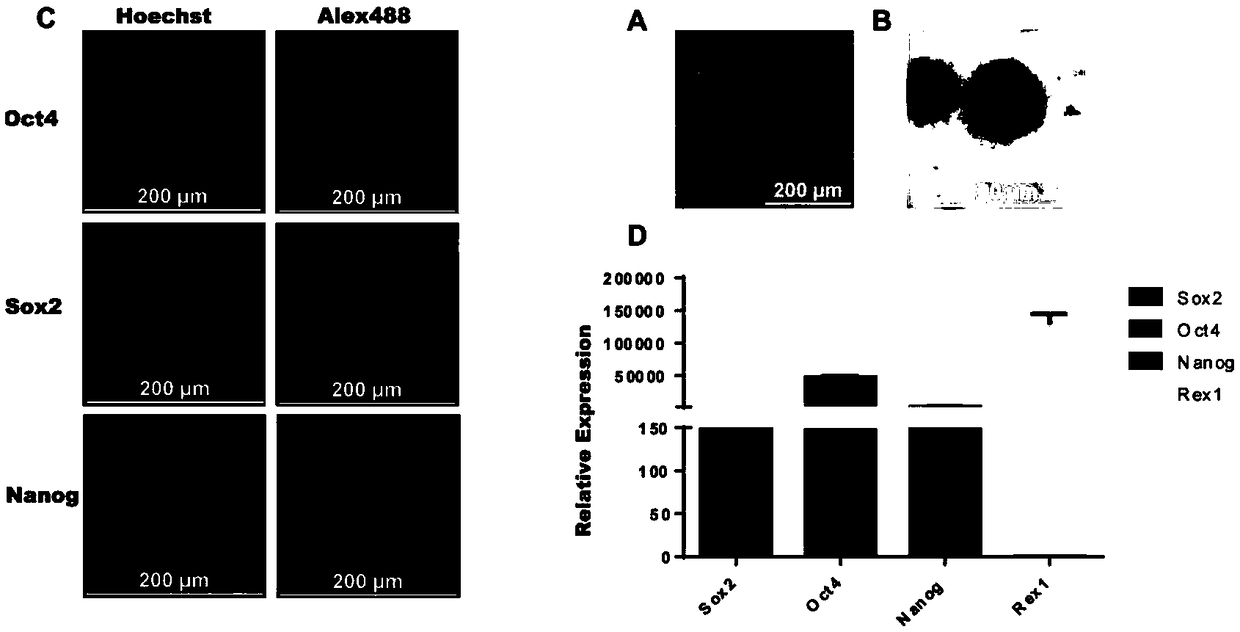
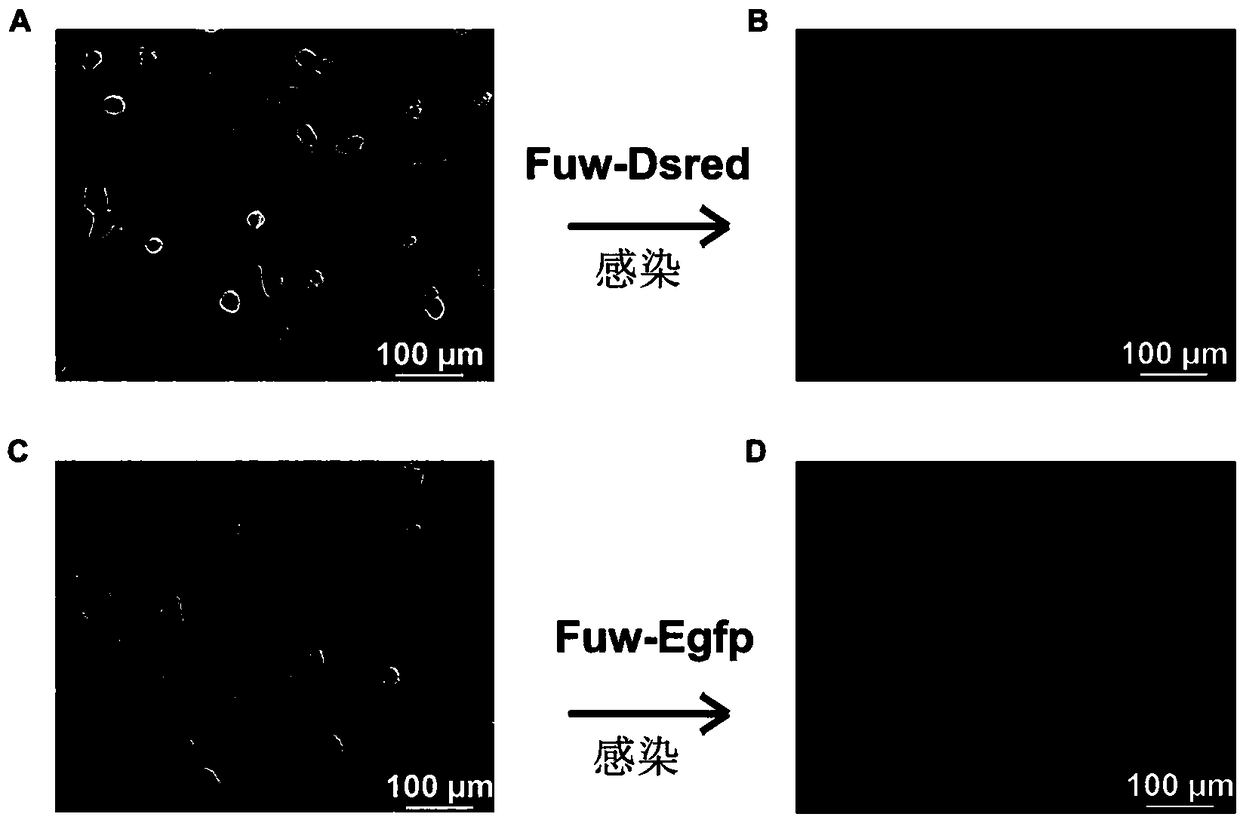
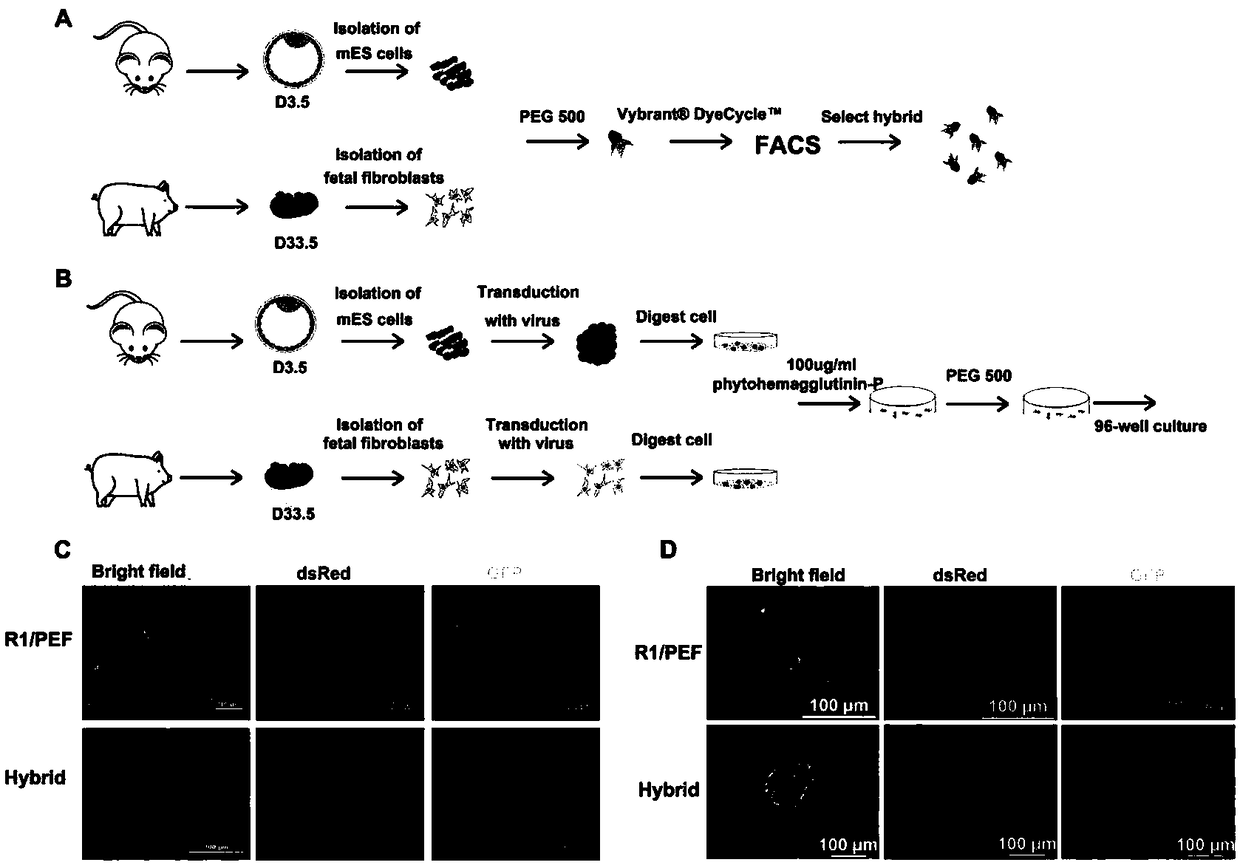
The invention belongs to the technical field of cell engineering and discloses a unicellular fusion method for pig and mouse cells. The unicellular fusion method is provided aiming at the problem of current low xenogenic cell fusion efficiency. The method includes: taking mouse embryonic stem cells and pig pluripotent stem cells or pig fibroblast cells as cellular fusion objects; adopting digestive juice containing pancreatin in mass fraction of 0.25% to digest the pig and mouse cells into single cells, culturing, and adopting cell agglutinin for constructing one-to-one unicellular pairs; culturing for 1min in polyethylene glycol in mass fraction of 50%, and continuing cell culture for seven days to obtain fused cells of pig and mouse single cells. The unicellular fusion method is applicable to animal breeding research or monoclonal antibody production.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Affinity purification method for reducing content of host cell protein in production of anti-human interleukin-33 monoclonal antibody
PendingCN113912728AImprove stabilityHigh yieldImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsComplementarity determining regionMonoclonal antibody agent
The invention discloses an affinity purification method for reducing the content of host cell protein in production of an anti-human interleukin-33 monoclonal antibody, which comprises the following steps: balancing before loading: balancing an affinity chromatography medium by using a buffer solution I to obtain the balanced affinity chromatography medium; loading a sample: combining the anti-human interleukin-33 monoclonal antibody fermentation liquor with the balanced affinity chromatography medium; and elution: carrying out pre-elution and final elution on the mixed solution so as to remove host cell protein and obtain the purified anti-human interleukin-33 monoclonal antibody, wherein the anti-human interleukin-33 monoclonal antibody comprises three heavy chain complementarity determining regions and three light chain complementarity determining regions, the three heavy chain complementarity determining regions are CDR-H1, CDR-H2 and CDR-H3, and the three light chain complementarity determining regions are CDR-L1, CDR-L2 and CDR-L3. The method is simple and easy to implement, amplification purification production can be carried out, the cell fermentation supernate does not need to be pretreated, the elution sample yield is high, the HCP residual quantity is kept at a low level, and therefore the pressure for removing HCP in the subsequent purification step is relieved.
Owner:QYUNS THERAPEUTICS CO LTD
Deoxynivalenol detection method based on single chain antibody, and kit
InactiveCN101004417BOvercome time-consuming, labor-intensive and expensive deficienciesKeep aliveMaterial analysisSnow moldSingle-Chain Antibodies
A method for detecting deoxy snow mold fusarine enol based on single chain antibody includes using separated single chain antibody of snow mold fusarine enol to detect relevant toxin deoxy snow mold fusarine enol and applying competitive enzyme-linked immunosorbent analysis to detect deoxy snow mold fusarine enol content in detected sample. The kit used for realizing said method is also disclosed.
Owner:FUJIAN AGRI & FORESTRY UNIV +1
Screening method of single antigen specific transgenic hybridoma cells
PendingCN114134163ARealize screeningStrong specificityAntibody mimetics/scaffoldsTissue cultureType specificCell membrane
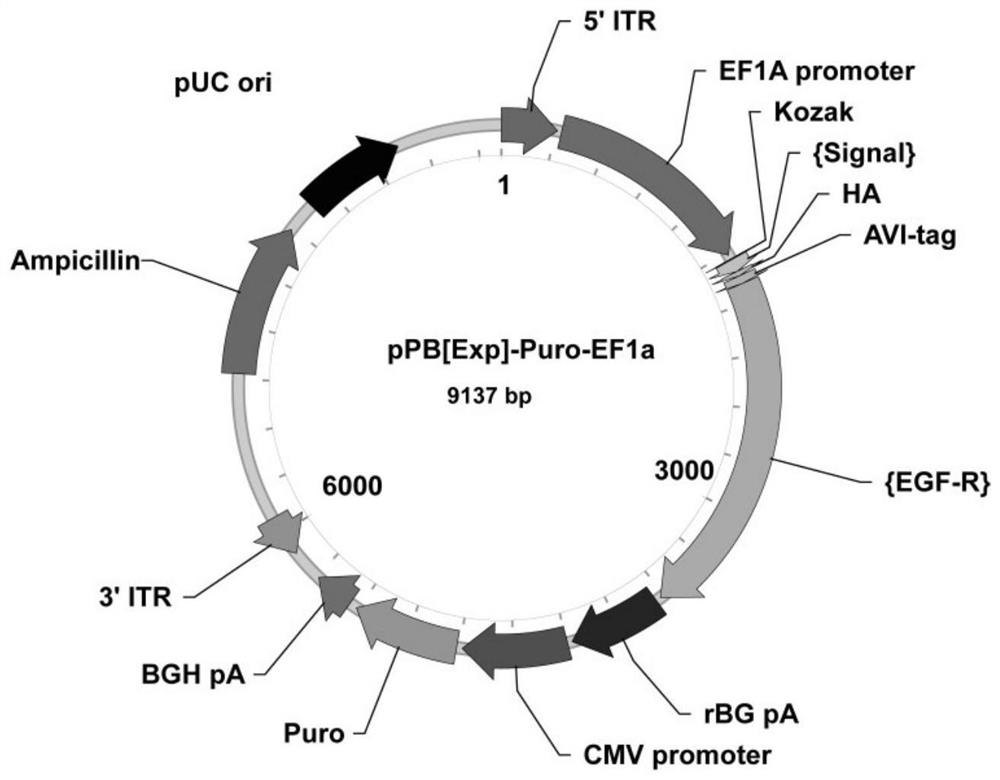
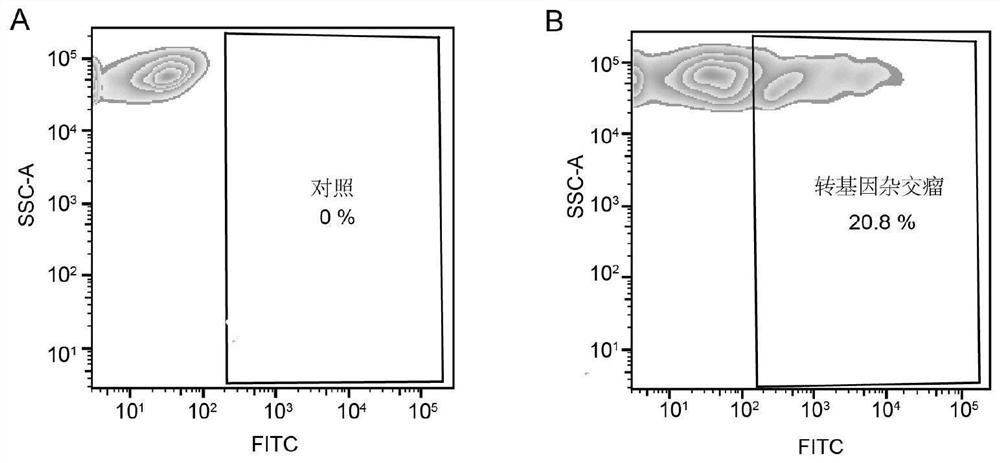
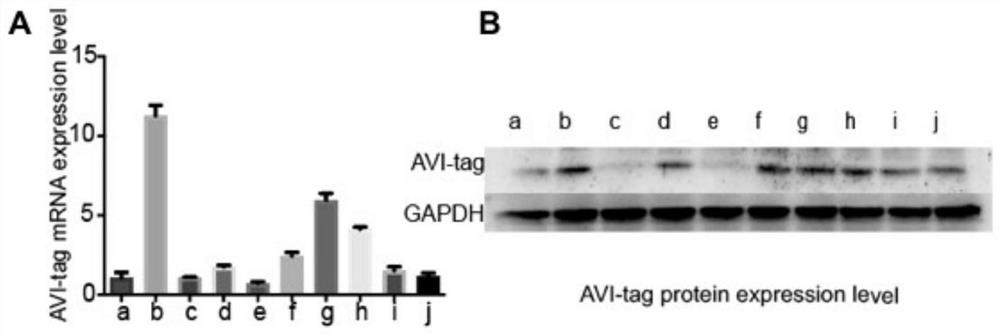
The invention discloses a single antigen specific transgenic hybridoma cell screening method, which comprises the following steps: preparing a transgenic Sp2 / 0 myeloma cell expressing EGF-R-AVI-tag fusion protein, and combining with a splenocyte fusion technology of a traditional hybridoma technology to obtain a transgenic hybridoma cell capable of displaying a secreting type specific antibody on a cell membrane. And screening of a single specific transgenic hybridoma cell is realized by using a single cell sorting system. The invention provides an accurate and high-throughput transgenic hybridoma monoclonal antibody production platform based on a transgenic technology. Compared with the traditional hybridoma technology, the preparation time and cost of a single antigen-specific hybridoma cell are remarkably shortened, the single cell rate and positive rate are improved, and the titer and affinity of the prepared monoclonal antibody are also remarkably improved. The transgenic hybridoma antibody preparation technology provided by the invention can be used for preparing rare monoclonal antibodies with high specificity, high sensitivity and high stability, and has a wide application prospect.
Owner:CHINA AGRI UNIV
Gene sequence capable of preventing gradual inactivation of promoter in subculture and applications of gene sequence
InactiveCN105255867AInactivation will notReduce yieldImmunoglobulinsVector-based foreign material introductionNucleotideNucleotide sequencing
The invention relates to molecular biology and particularly discloses a gene sequence capable of preventing gradual inactivation of a promoter in subculture and applications of the gene sequence. The nucleotide sequence of the gene sequence is shown as SEQ ID No.1. The gene sequence is used for constructing a carrier and a CHO cell line, can prevent gradual inactivation of the promoter in subculture of CHO cells and can be used for monoclonal antibody production through the CHO cell line to increase the monoclonal antibody yield.
Owner:BEIJING YISHENGHE BIOLOGICAL SCI & TECHCO LTD
A method and application of increasing monoclonal antibody production
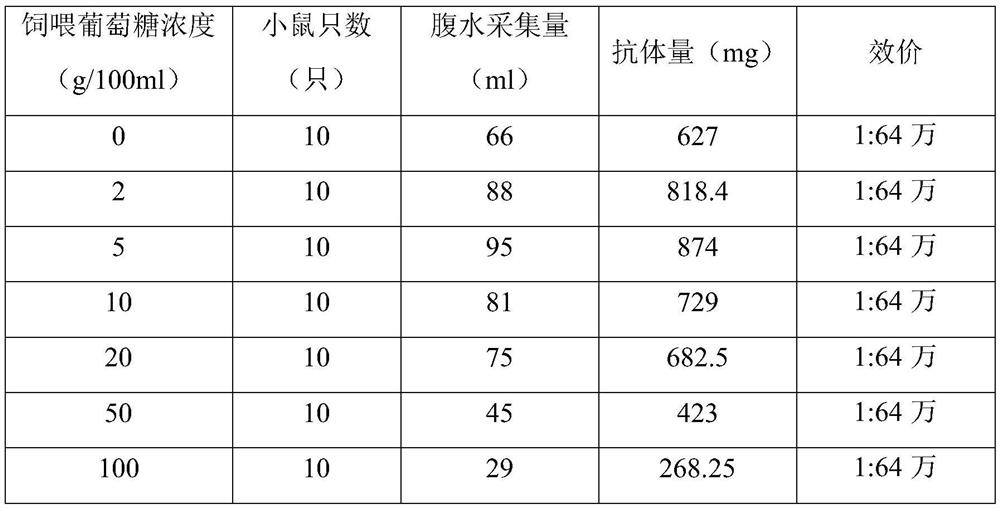
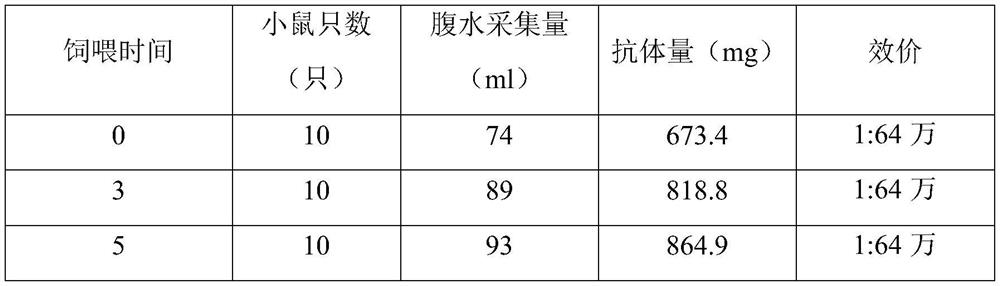
The invention provides a method for increasing the production of monoclonal antibodies, comprising the following steps: (1) cloning and culturing hybridoma cells, and injecting the cultured hybridoma cells into animals; (2) feeding glucose solution to animals (3) collecting ascites antibody; (4) purifying the collected ascites antibody. In the present invention, after the animals are injected into the hybridoma cells, the mice start to be fed with the glucose solution mixed with pure water, and unexpected technical effects are obtained. The monoclonal antibody obtained by the method is equivalent, greatly reduces the cost of antibody production, greatly increases its economic benefits, and provides a new idea for the supply of raw materials in the IVD industry.
Owner:ZYBIO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com