Screening method of single antigen specific transgenic hybridoma cells
A hybridoma cell and transgenic technology, applied in chemical instruments and methods, biochemical equipment and methods, specific peptides, etc., can solve the problems of normal function and vitality damage, fat chain instability, loss, etc., to improve the rate of single cells and positive rate, high sensitivity, promising effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044]The preparation method of transgenic Sp2 / 0 myeloma cells is as follows: the signal peptide of EGF-R is inserted into the N-terminus of the bioreceptor peptide AVI-tag sequence, and the transmembrane region of EGF-R is inserted into the C-terminus. Transfected into the genome of Sp2 / 0 myeloma cells, screened with cell culture medium containing 10% (v / v) FCS, 2mM glutamine, and 2μg / mL puromycin for 21 days, and used conventional complete medium to counteract purine-resistant Myeloma transgenic Sp2 / 0 myeloma cells were expanded and cultured.
[0045] The screening method of a single transgenic Sp2 / 0 myeloma cell is as follows: first, the transgenic Sp2 / 0 myeloma cell is biotinylated in vitro, and the transgenic Sp2 / 0 myeloma cell is treated with 2% BSA and 2mM PE-streptavidin conjugate (PE is fluorescein , that is, phycoerythrin (P-phycoerythrin, PE)) resuspended biotinylated transgenic Sp2 / 0 myeloma cells in PBS, incubated at 4°C for 20 minutes, washed 3 times with PBS, an...
Embodiment 1
[0053] Embodiment 1 Preparation of transgenic Sp2 / 0 myeloma cells
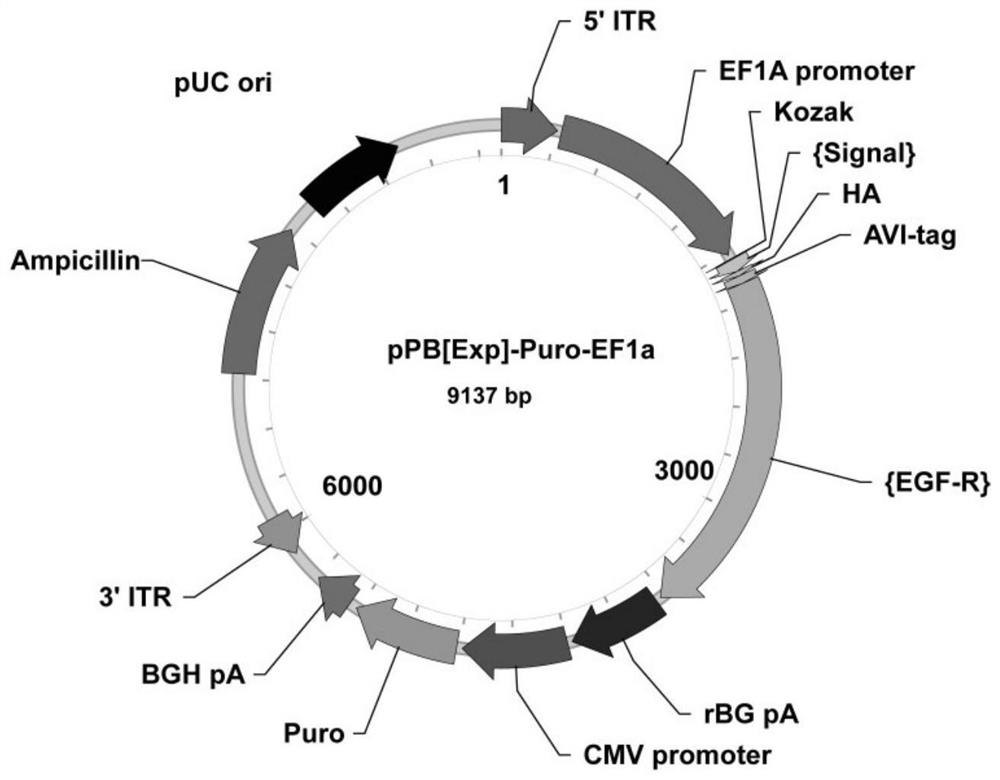
[0054] 1. Insert the signal peptide of EGF-R (amino acids 1-24) at the N-terminus of the biotin receptor peptide (AVI-tag) sequence, and insert the transmembrane of EGF-R (amino acids 25-668) at the C-terminus area( figure 1 ). The amino acid sequence of the EGF-R-AVI-tag fusion protein is shown in SEQ ID NO:1.
[0055] 2. One day before transfection, 0.5×10 5 -1.0×10 5 Sp2 / 0 myeloma cells were plated in a 6-well plate to achieve an optimal cell density of 70-80%.
[0056] 3. On the day of transfection, discard the medium supernatant, add 2 mL of complete medium, mix 0.2 μg transposase plasmid (mPB, helper plasmid) and 2 μg AVI-tag-EGF-R plasmid (pPB EF1a, donor plasmid) , dilute to 100 μL of Opti-MEM buffer, add Reagent, incubated at room temperature.
[0057] 4. Add the incubated solution to the Sp2 / 0 myeloma cells drop by drop. After 18-24 hours, add the selection medium containing 2 μg / mL puromycin...
Embodiment 2
[0058] Example 2 Screening of a single transgenic Sp2 / 0 myeloma cell
[0059] 1. Put 4×10 6 transgenic Sp2 / 0 myeloma cells with optimized 5mM MgCl 2 , 10 mM ATP, 5 μM biotin and 0.85 μg BirA in PBS, at 37 °C, 5% CO 2 Incubate under certain conditions for 30 min to achieve in vitro biotinylation of transgenic Sp2 / 0 myeloma cells.
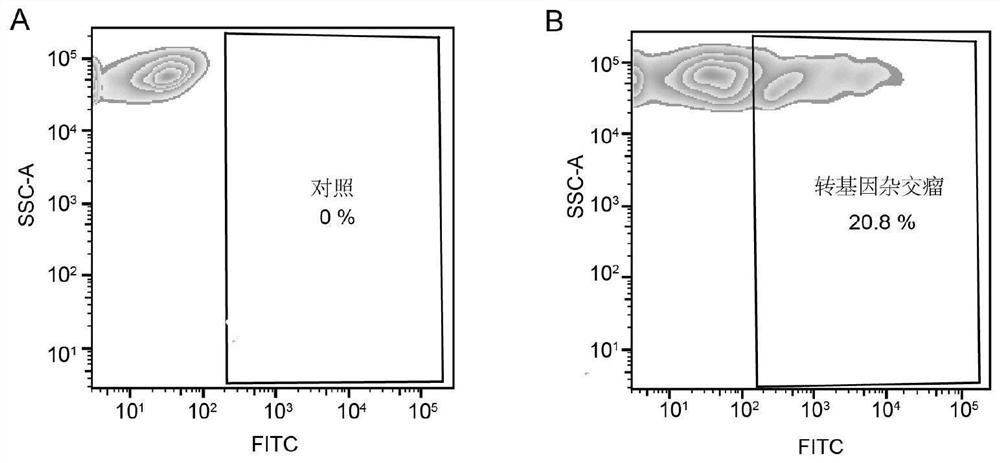
[0060] 2. Use 5mM MgCl 2 Wash the biotinylated transgenic Sp2 / 0 myeloma cells with PBS, resuspend the cells in PBS containing 2% BSA, add 2mM PE-streptavidin conjugate, incubate at 4°C for 20 minutes, and enrich by FACS and preparation of a single PE-streptavidin-labeled transgenic Sp2 / 0 myeloma cell ( figure 2 , A and B).
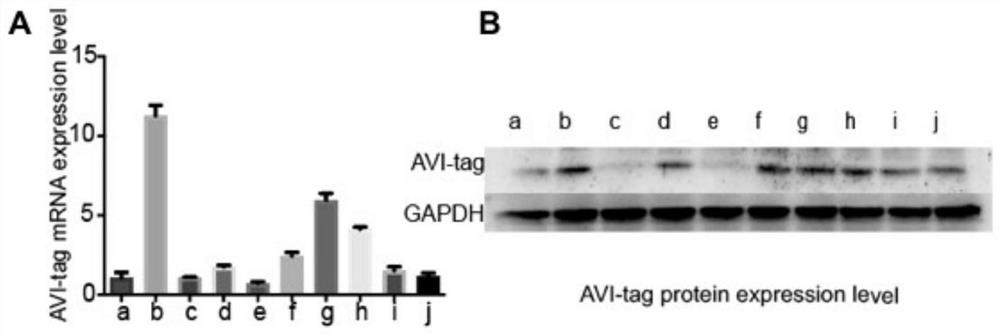
[0061] The top ten transgenic Sp2 / 0 myeloma cells with good growth status were selected, and the foreign protein core element AVI-tag on the surface of the transgenic Sp2 / 0 myeloma cells was analyzed by real-time fluorescent quantitative PCR technology and Western blotting. mRNA and protein expression, so as to select the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com