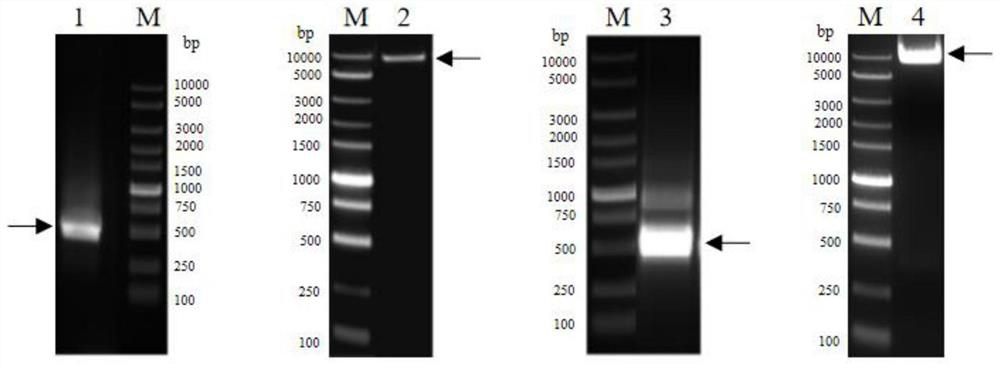
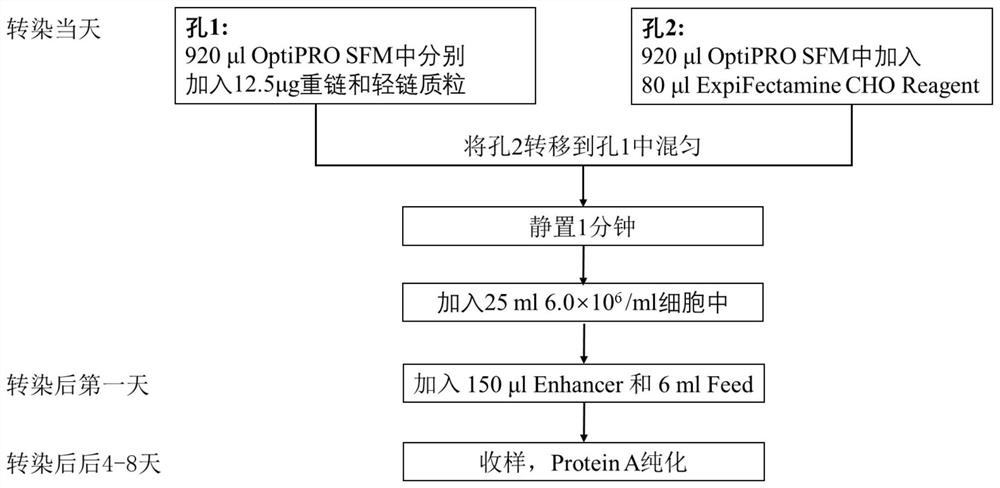
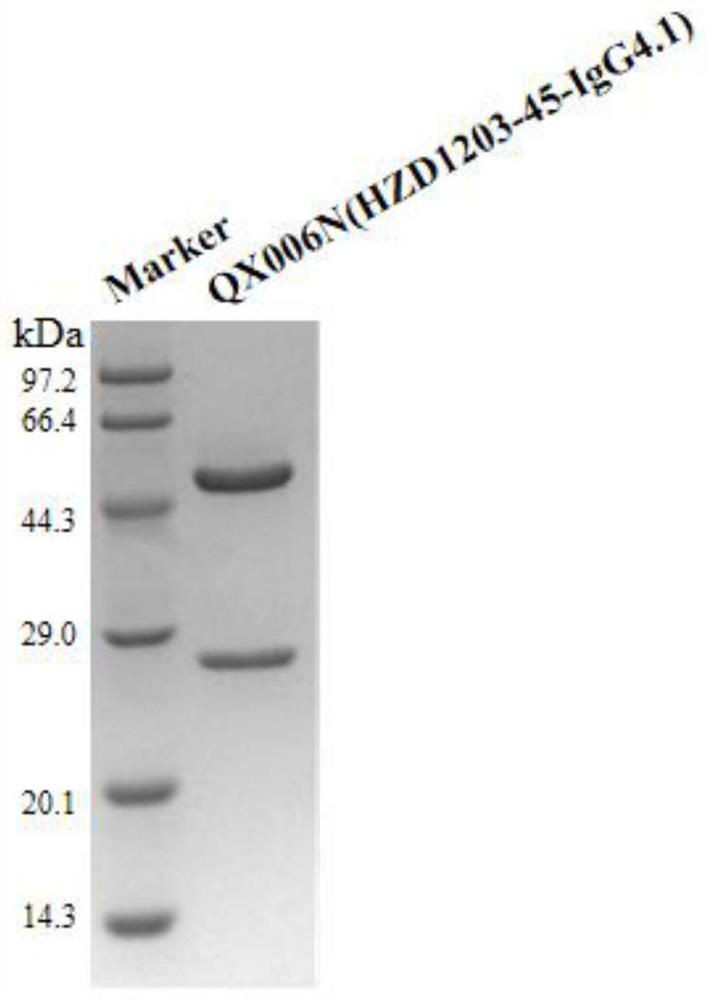
Affinity purification method for reducing content of host cell protein in monoclonal antibody production
A host cell protein and monoclonal antibody technology, applied in the biological field, can solve the problems of host cell protein residue and amplification, and achieve the effect of simple and easy affinity process and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1 Preparation of anti-human interferon alpha receptor 1 monoclonal antibody QX006N
[0122] Purchasing human interferon alpha receptor 1 (IFNAR1) from Shanghai Nearshore Technology Co., Ltd. for immunization of New Zealand rabbits, using B cell cloning technology to obtain antigen-binding specific antibody clones, and then screening for binding to human IFNAR1 and having human IFNAR1 inhibitory activity of monoclonal antibodies. First, the cell supernatant was detected by Binding ELISA, and the clones binding to human IFNAR1 were selected; then, the clones with human IFNAR1 inhibitory activity were selected for detection by HEK Blue IFNα / β reporter gene cell method. The above immunization and screening processes are entrusted to commercial companies.
[0123] 37 clones were selected for recombinant expression and sequenced. It was determined that 362# and 1203# had the best cell neutralizing activity, and the sequences of the two clones were very similar. The...
Embodiment 2
[0129] Embodiment 2 Equilibrium dissociation constant (K D ) determination
[0130] The affinity between QX006N (HZD1203-45-IgG4.1) and human IFNAR1 was detected by BiacoreT200, and all processes were carried out at 25°C. Using a commercial Protein A chip, an appropriate amount of antibody was immobilized by the capture method, so that the Rmax was around 50RU, and the capture flow rate was 10 μl / min. The antigen was serially diluted, the flow rate of the instrument was switched to 30 μl / min, and the concentration flowed through the reference channel and the channel of the immobilized antibody in order of concentration from low to high, and the buffer was used as a negative control. After each association and dissociation, the chip was regenerated with pH 1.5 glycine. Use the analysis software that comes with the instrument to select the 1:1 binding model in the Kinetics option for fitting, and calculate the binding rate constant k of the antibody a , the dissociation rate ...
Embodiment 3
[0135] Example 3QX006N and Anifrolumab neutralize human interferon-induced HEK Blue IFNα / β cell STAT1 / 2 phosphorylation activity
[0136] Use the HEK Blue IFNα / β reporter gene cell line to measure the phosphorylation activity of the intracellular signal molecule STAT1 / 2 mediated by QX006N antagonizing interferon through IFNAR1: the cells in the culture medium were divided into 4×10 per well 4 Cells were added to 96 wells and then incubated at 37°C and 5% CO 2 conditions overnight. Serial dilutions of antibody concentrations ranging from 0 to 5 μg / ml were added to the cells along with 0.2 ng / ml of IFNα.2b. Then at 37 °C and 5% CO 2 Cultivate under conditions for 24 hours, collect the cell culture supernatant and add 10% QUANTI-Blue TM Detection reagents at 37°C and 5% CO 2 React under the conditions for 1 hour, then detect the OD630nm value and draw the dose-effect curve, and then analyze the antagonistic activity of the antibody. The experimental results show that QX006N c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com