Application of flavonoids
A technology of flavonoids and compounds, applied in the application field of flavonoids, can solve the problems of cytotoxicity, low blood drug concentration, narrow anti-tumor spectrum, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
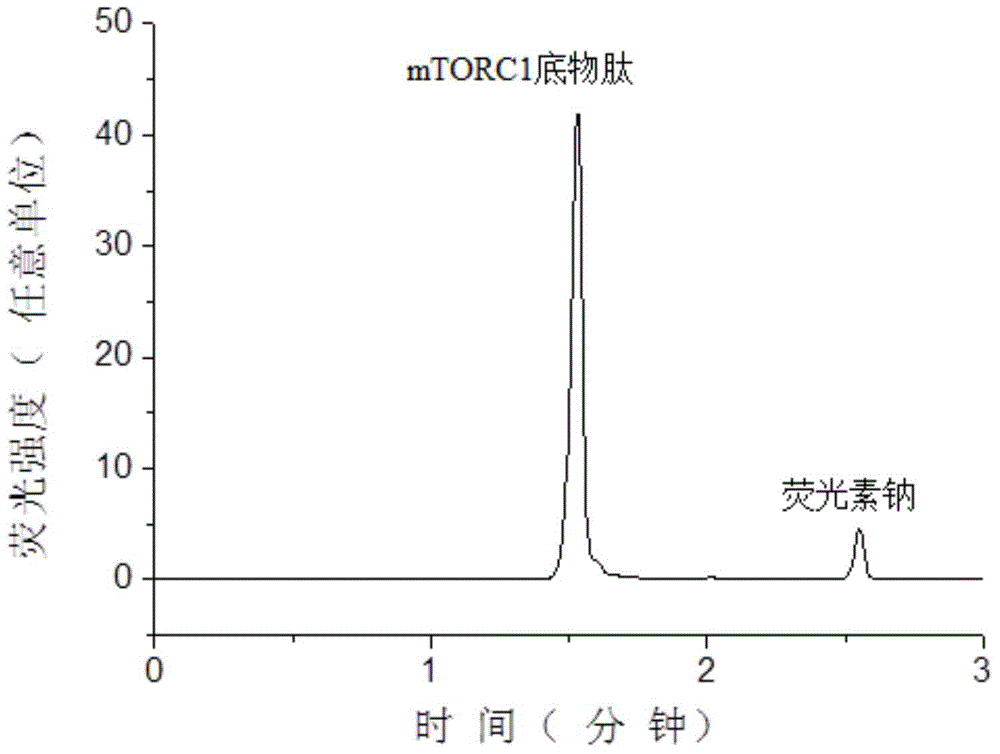
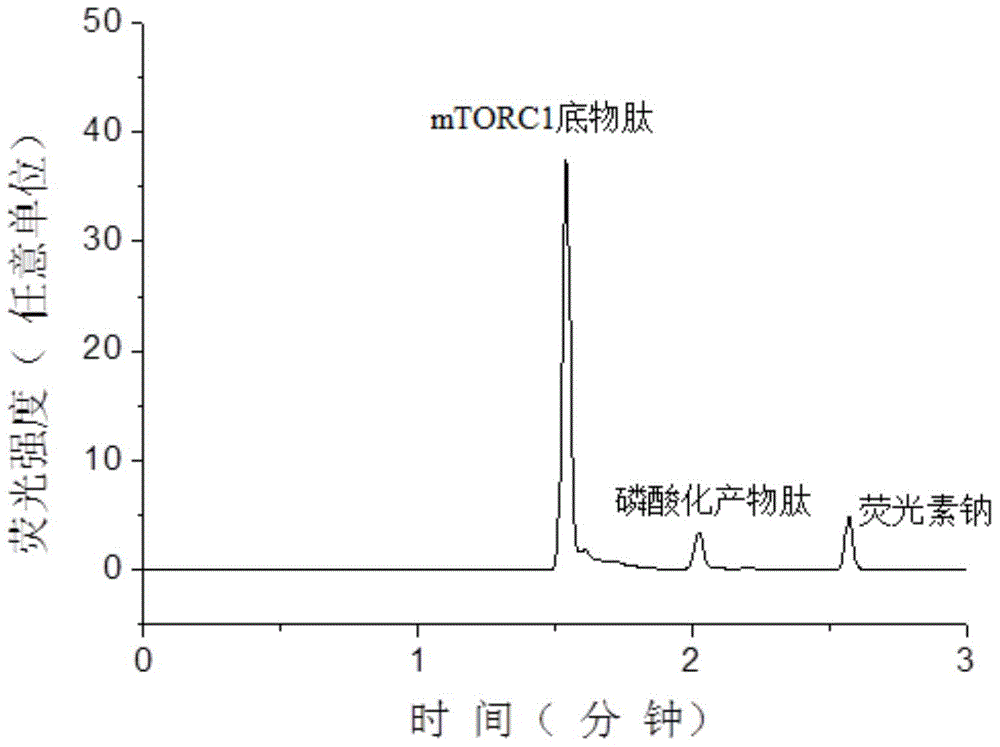
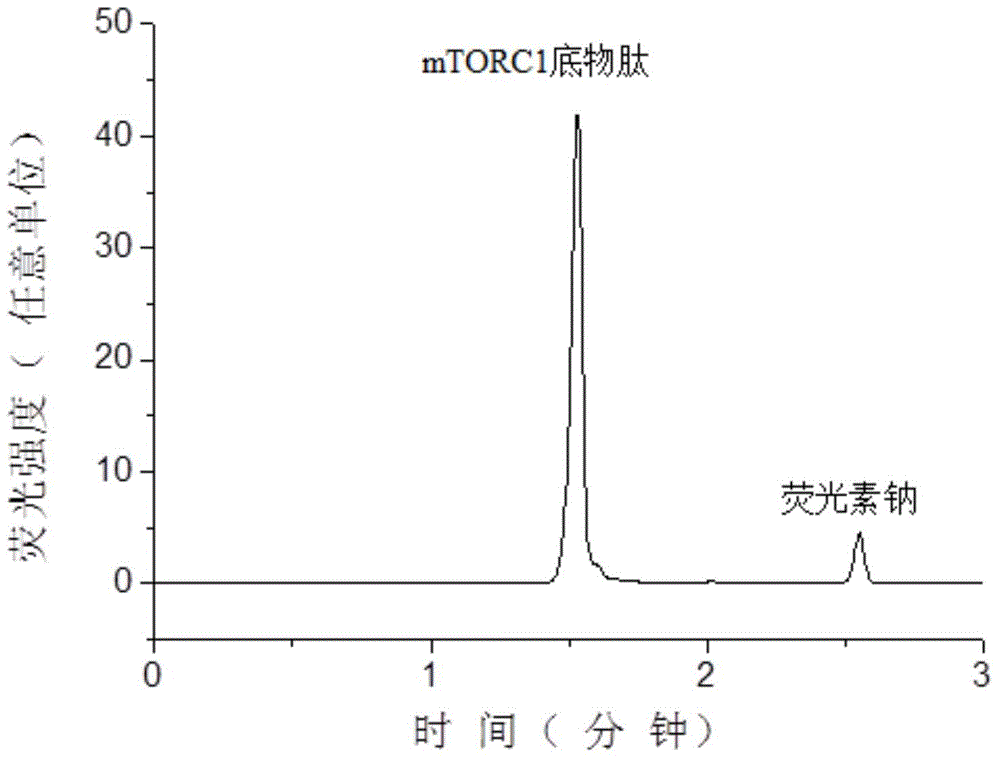
[0059] Substrate peptide STTPGGTLFSTTPG (the sequence of the substrate peptide is shown in SEQIDNO: 1 in the sequence listing, referring to the mTOR kinase test method of PerkinElmer Company), the substrate peptide was synthesized by Jill Biochemical (Shanghai) Co., Ltd., and its N 5-carboxyfluorescein (5-FAM) labeling is carried out at the end to facilitate fluorescence detection and improve the sensitivity of detection. The peptide structure of the obtained substrate peptide is shown in formula II:
[0060]
[0061]
[0062] The mTORC1 used in the examples was purchased from Sigma Reagent Co., Ltd., product number: SRP0364.
[0063] Prepare 25 mL of enzyme reaction buffer: 50 mM 4-hydroxyethylpiperazineethanesulfonic acid (Hepes), pH adjusted to 7.5 by 4M NaOH, 2 mM 1,4-dithiothreitol, 10 mM magnesium chloride, 1 mM ethylene glycol bis(2 -aminoethyl ether) tetraacetic acid, 0.01% (v / v, the same below) Tween 20 and 3mM manganese chloride.
[0064] Use 2 μL of enzyme re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com