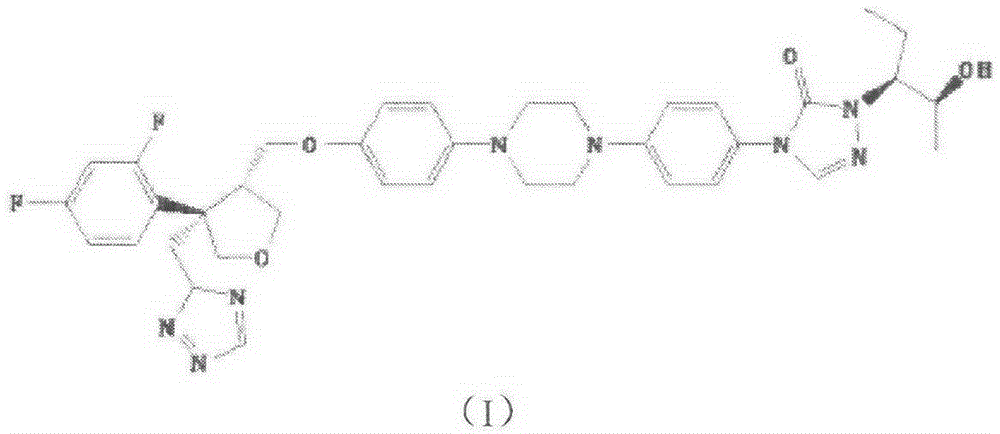
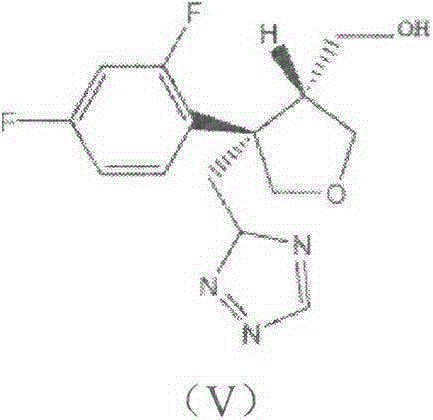
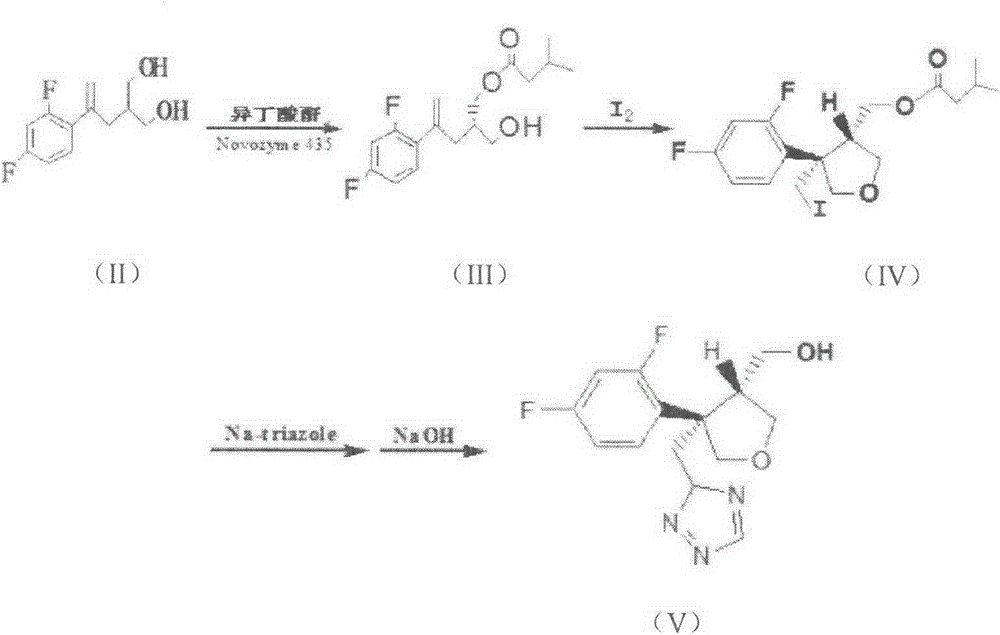
Preparation method of posaconazole intermediate
A technology for posaconazole and intermediates, which is applied in the field of medicinal chemistry synthesis, can solve the problems of expensive enzyme catalysts, complicated process operation, unfavorable production amplification, etc., and achieves the effects of reducing cost, simple operation process and improving catalytic efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Put 5.7 g of compound (II) into a conical flask, add 60 ml of phosphate buffer solution with pH=5.5, add 2.4 g of isobutyric anhydride, add 0.6 ml of Tween 80 liquid, stir well, add diatomaceous earth to immobilize lipase 100unit / mol, put the Erlenmeyer flask on a shaker, 37°C, 200r / min, and react for 24h. Filtration of immobilized lipase is treated and reused, and the filtrate is organically extracted and concentrated to obtain a mixture of posaconazole intermediates (II) and (III), separated by flash column chromatography to obtain posaconazole intermediate (III) 3.5g, the yield is about 47%, and the determined ee value is 99.1%. The NMR results are as follows:
[0028] 1H-NMR (400MHZ, CDCl3):
[0029] 7.53-7.46(m, 1H), 6.89-6.86(m, 1H), 6.83-6.78(m, 1H), 4.16-4.12(dd, 1H), 4.09-4.04(m, 2H), 3.87-3.84(dd , 1H), 3.69(s, 2H), 2.59-2.53(m, 3H), 2.19-2.12(dd, 1H), 1.18(s, 3H), 1.15(S, 3H).
[0030] MS=271M+
[0031] The reaction is shown in the following formula:
...
Embodiment 2
[0034] Put 5.7 g of compound (II) into a conical flask, add 60 ml of phosphate buffer solution with pH=6.5, add 2.4 g of isobutyric anhydride, add 1.2 ml of Tween 80 liquid, stir well, add diatomaceous earth to immobilize lipase 100unit / mol, the Erlenmeyer flask was placed on a shaker, 37°C, 200r / min, and reacted for 20h. Filtration of immobilized lipase is treated and reused, and the filtrate is organically extracted and concentrated to obtain a mixture of posaconazole intermediates (II) and (III), separated by flash column chromatography to obtain posaconazole intermediate (III) 3.2g, the yield is about 42.9%, and the determined ee value is 99.2%.
Embodiment 3
[0036] Put 5.7 g of compound (II) into a conical flask, add 60 ml of phosphate buffer solution with pH=7.0, add 2.4 g of isobutyric anhydride, add 1.8 ml of Tween 80 liquid, stir well, add diatomaceous earth to immobilize lipase 50unit / mol, put the Erlenmeyer flask on a shaker, 35°C, 200r / min, and react for 24h. Filtration of immobilized lipase is treated and reused, and the filtrate is organically extracted and concentrated to obtain a mixture of posaconazole intermediates (II) and (III), separated by flash column chromatography to obtain posaconazole intermediate (III) 3.7g, the yield is about 49.6%, and the determined ee value is 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com