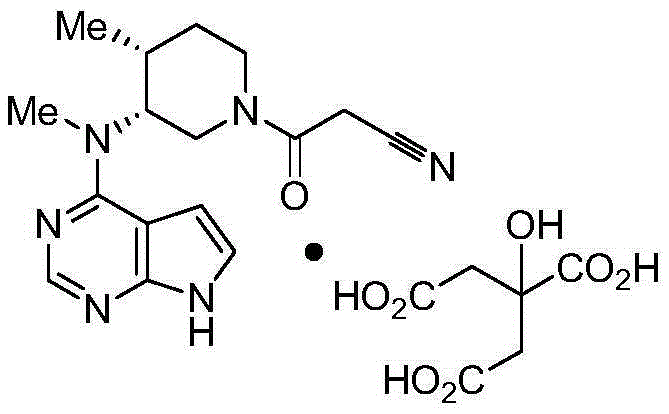
Application of tofacitinib citrate and medicinal composition thereof in preparation of medicament for treating sjogren's syndrome
A technology of Sjogren's syndrome and tofacitinib, applied in the preparation of drugs for the treatment of Sjögren's syndrome, in the field of tofacitinib citrate and its composition, to achieve the effect of improving the symptoms of dry mouth and eyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of tablet (5mg / tablet)
[0032] formula:
[0033] Tofacitinib citrate 8g (equivalent to containing Tofacitinib 5g)
[0034] Hydroxypropyl cellulose 25g,
[0035] Microcrystalline cellulose 30g,
[0036] Lactose 30g,
[0037] Made in 1000 pieces.
[0038] Preparation method: Weigh the raw materials and auxiliary materials of the above formula, add them to a three-dimensional mixer and mix for 15 minutes, then add 10 g of magnesium stearate, and then mix with a three-dimensional mixer for 5 minutes to obtain the total blend granules. The mixed granules are compressed into tablets containing 8 mg of tofacitinib citrate (equivalent to 5 mg of tofacitinib) per tablet. The weight of the tablet is about 103 mg, and the hardness is controlled at 8-10 kg.
Embodiment 2
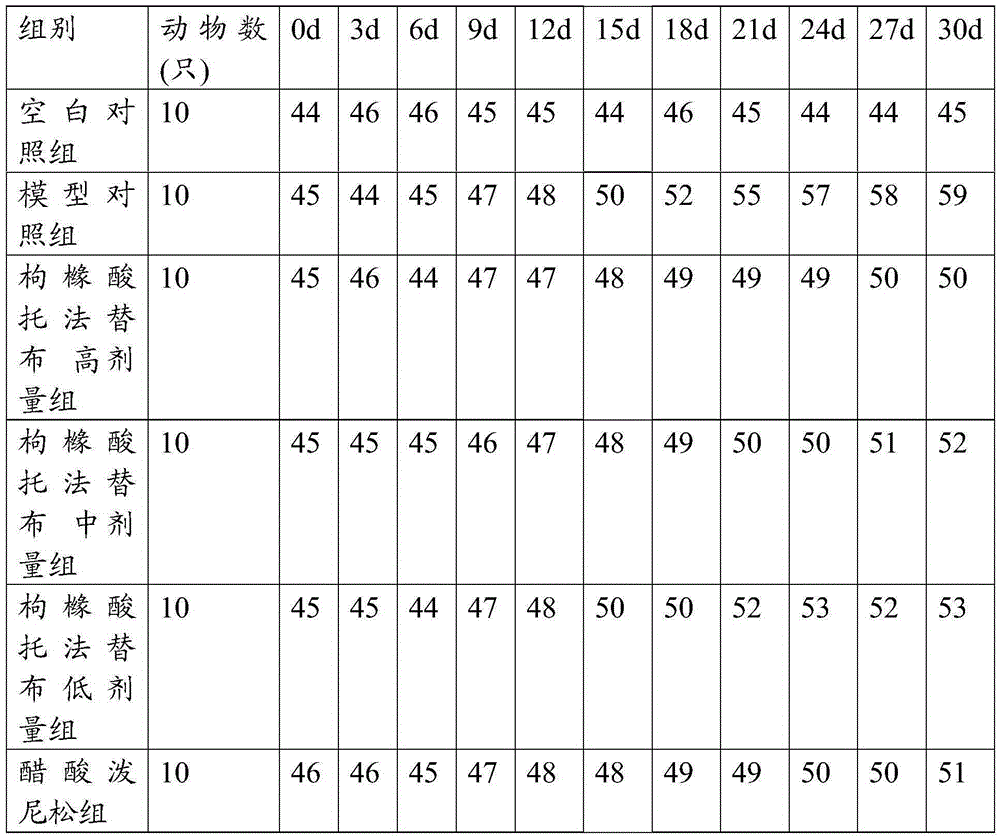
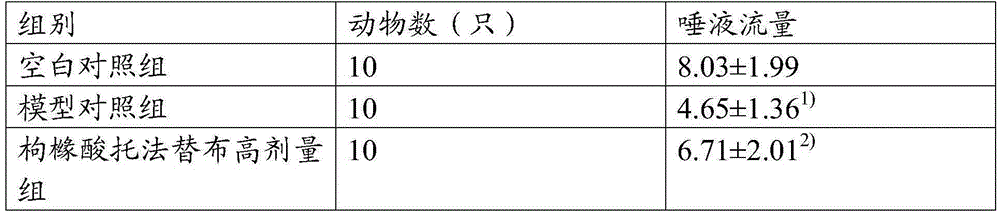
[0039] Embodiment 2: Administration of SS mouse model (tofacitinib citrate)
[0040] 1. Dose conversion: refer to the equivalent dose ratio table between humans and animals based on body surface area conversion, tofacitinib is administered 1-20 mg per day, converted to 0.15-3 mg / kg for mice; prednisone acetate per adult The daily dosage is 20mg, converted into a mouse dosage of 3.0mg·kg -1 , generally calculated on the basis of an adult body mass of 60kg.
[0041] 2. Experimental animals: select 40 BALb / C inbred mice aged 8 weeks, SPF grade, male or female, with a body weight of about 20 g. Strictly follow the "3R" principle, the experimental animal breeding room has natural light, the temperature is controlled at 18-19°C, the humidity is controlled at 40%-70%, and the animals are free to eat and drink. At the end of the experiment, the animals were killed by necking.
[0042] 3. Main drugs and reagents:
[0043] Tofacitinib citrate high dose group: 3mg
[0044] Tofacitin...
Embodiment 3
[0066] Example 3 case
[0067] Select 56 cases of Sjögren's syndrome patients. Among them, there were 50 females and 6 males, with an average age of (35±12) years. The course of the disease ranges from 6 months to 45 months. The diagnostic criteria are in accordance with the International Classification (Diagnosis) of Sjogren's Syndrome (ARA, 1993).
[0068] 3.1 Method of taking medicine
[0069] An open trial was adopted without a control group. Tofacitinib citrate 5mg, orally once a day, for 36 weeks.
[0070] 3.2 Observation indicators
[0071] Erythrocyte sedimentation rate (ESR), blood routine, urine routine, rheumatoid factor, liver function, kidney function, immunoglobulin (IgG, IgM, IgA), salivary gland ECT, Schirmer test, before taking the medicine, 12, 24 and 36 weeks after taking the medicine. Serum protein electrophoresis and 10cm visual analog ruler method were used to measure the evaluation of the degree of disease by doctors and patients respectively. Sec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com