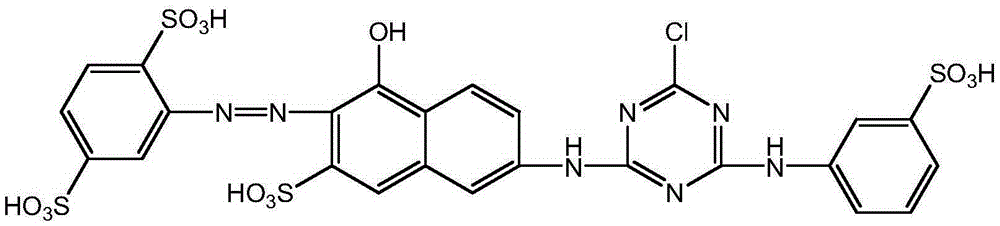
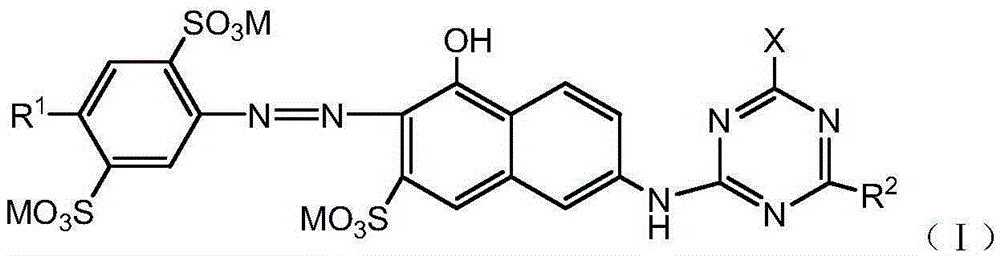
Orange active dye compound, and preparation method and applications thereof
A technology for reactive dyes and compounds, which is applied to orange reactive dye compounds, a preparation method thereof, and an application field in cellulose fiber printing and dyeing, and achieves the effects of novel structure, good binding stability and excellent performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1. A contraction reaction
[0047] Weigh 18.4g (0.1mol) of cyanuric chloride, 30mL of bottom water, 100g of ice, and 0.5g of MF for beating for 20min. Weigh 23.9g (0.1mol) of J acid and 250mL of bottom water, adjust the pH to 6.5-7.0 with 30% liquid caustic soda, add dropwise to the cyanuric chloride solution, and control the pH to 2.0-3.0 during the dropwise addition. Temperature 0-5°C. After the addition, adjust the pH to 3.0-3.5 with sodium bicarbonate, react at a temperature of 0-5°C for 2-3 hours, then detect with ethanol solution of 4-dimethylaminobenzaldehyde, the microscopic yellow is the end point, and a shrinkage solution is obtained ,spare.
[0048] 2. Diazo reaction
[0049] Weigh 10.7g (0.1mol) of p-methylaniline and add it dropwise to 24g of 98% sulfuric acid (containing 0.24mol of sulfuric acid) at a temperature of 50-55°C, stir for 30min, and add 32g of 66% oleum dropwise within 2-3h (SO 3The content is 0.26mol), and the temperature is controlled at...
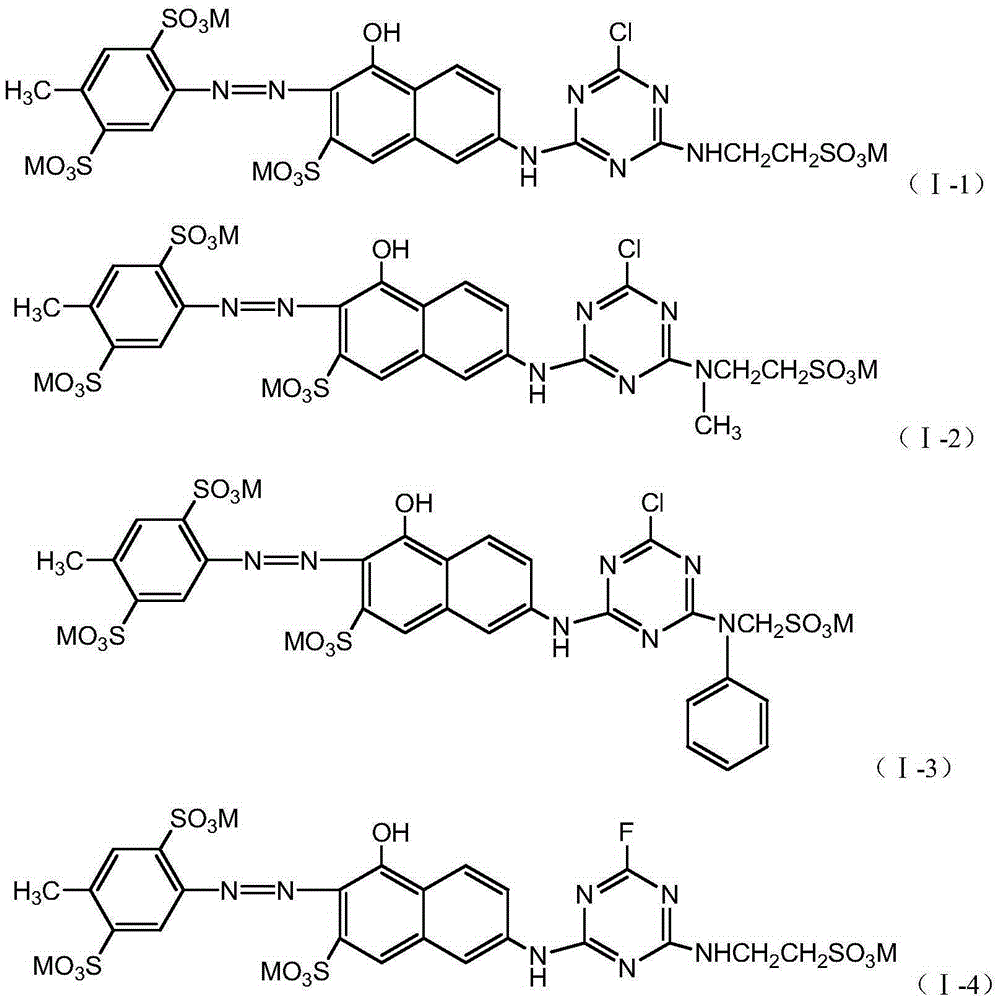
Embodiment 2~6
[0057] According to the preparation method in Example 1, different coupling components are used to prepare, and the dye compounds with the structures shown in the following table 1 can be prepared respectively:
[0058] Table 1
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com