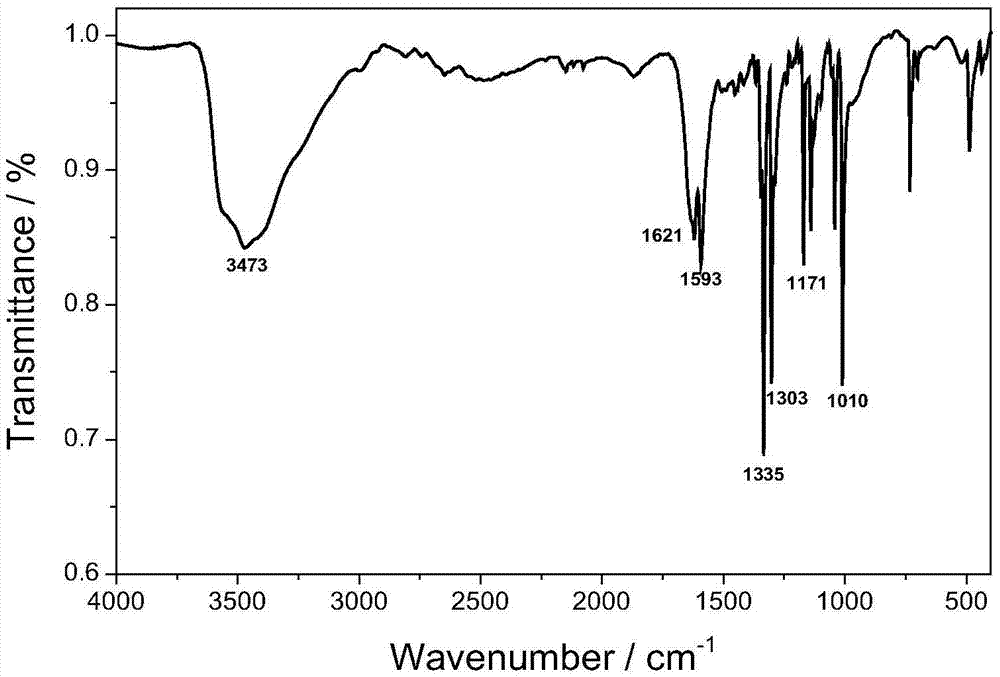
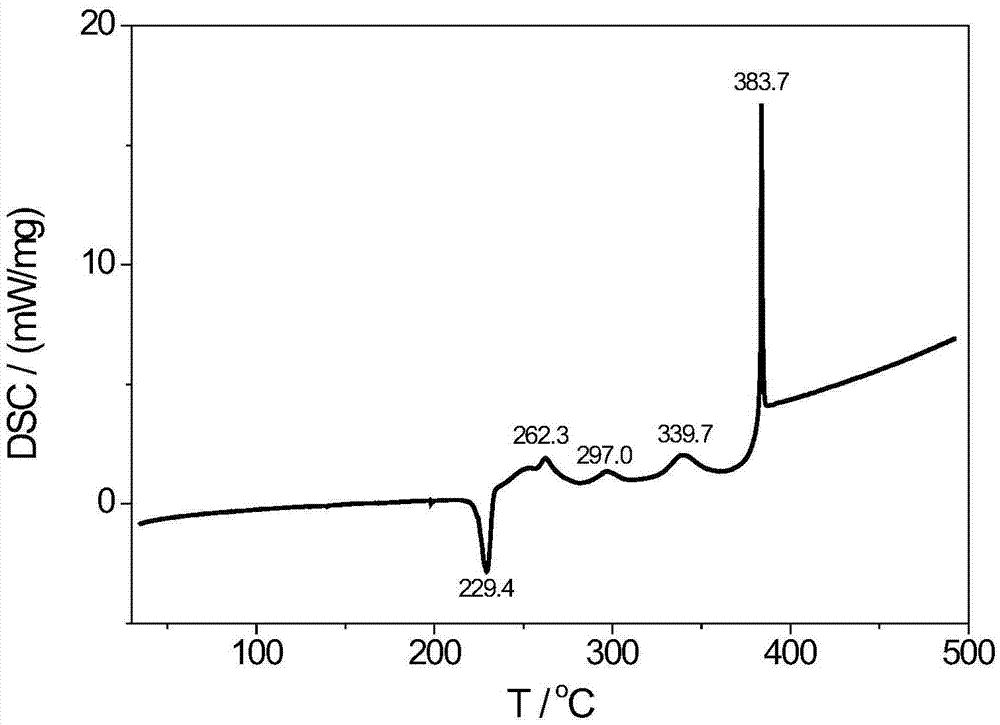
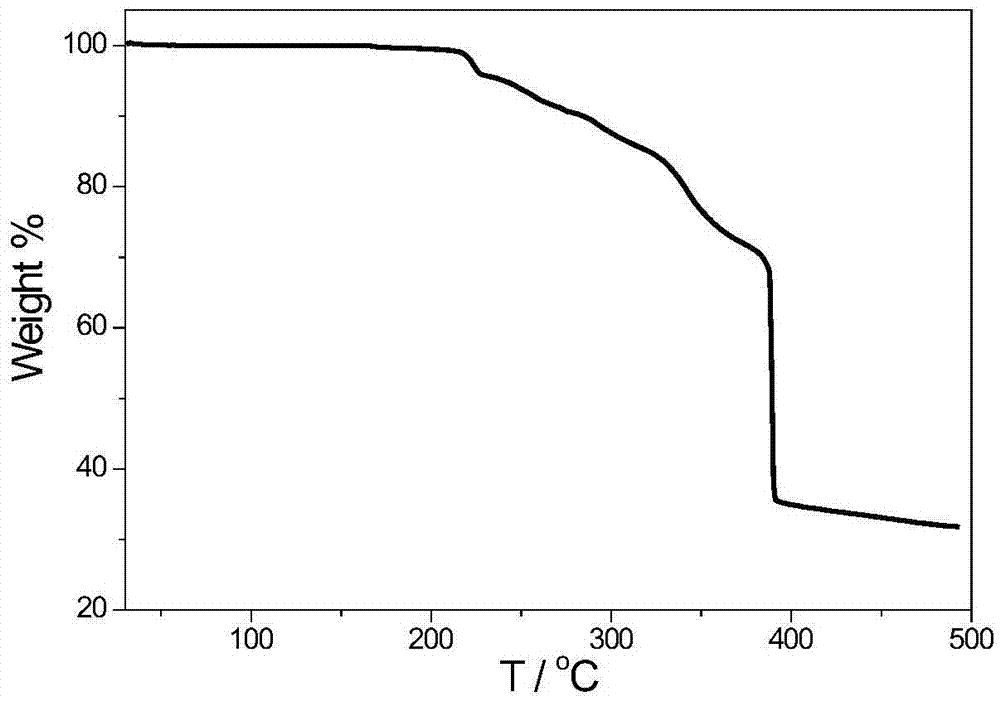
1,1'-dihydro-5,5'-bistetrazole-lead coordination polymer and preparation method thereof
A technology of coordination polymer and bitetrazole, which is applied in the field of 1,1'-dihydro-5,5'-bitetrazole-lead coordination polymer and its preparation, can solve the problem of poor catalytic effect and carbon content high, does not meet the requirements of the development of smoke-free low characteristic signal propellants, and achieves the effects of reduced friction sensitivity, mild experimental conditions, and simple and easy preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Dissolve 0.14g (1mmol) of 1,1'-dihydro-5,5'-bitetrazol in 4ml of DMF / water (1:3) mixed solvent, and filter it into a 50ml beaker. 0.331g (1mmol) of lead nitrate was dissolved in 6ml of water and filtered into the same beaker. Heat to 80°C and hold for 10 hours. Then the temperature was lowered to 30°C at a rate of 3°C / hour. The resulting colorless crystals were collected by suction filtration and dried in the air. The yield was 40%.
Embodiment 2
[0018] Dissolve 0.14g (1mmol) of 1,1'-dihydro-5,5'-bitetrazol in 10ml of water, and filter it into a 20ml stainless steel reactor with Teflon liner. 0.331g (1mmol) of lead nitrate was dissolved in 10ml of water and filtered to the same reaction kettle. After the kettle body was sealed, the temperature was raised to 100° C. and maintained for 48 hours. Then the temperature was lowered to 30°C at a rate of 5°C / hour. The resulting colorless crystals were collected by suction filtration and dried in the air, with a yield of 42%.
Embodiment 3
[0020] Dissolve 0.14g (1mmol) of 1,1'-dihydro-5,5'-bitetrazol in 6ml of acetonitrile / water (1:2) mixed solvent, filter into 20ml of Teflon-lined Stainless steel reactor. 0.331g (1mmol) of lead nitrate was dissolved in 12ml of water and filtered to the same reaction kettle. After the kettle body was sealed, the temperature was raised to 130° C. and maintained for 48 hours. Then the temperature was lowered to 30°C at a rate of 6°C / hour. The resulting colorless crystals were collected by suction filtration and dried in the air, with a yield of 41%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com