Preparation and separation purification methods of drug intermediate
A product and compound technology, applied in the direction of organic chemistry, can solve the problems of three wastes discharge energy, long process cycle, waste, etc., and achieve the effect of saving energy and cost, saving process time, and reducing the discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
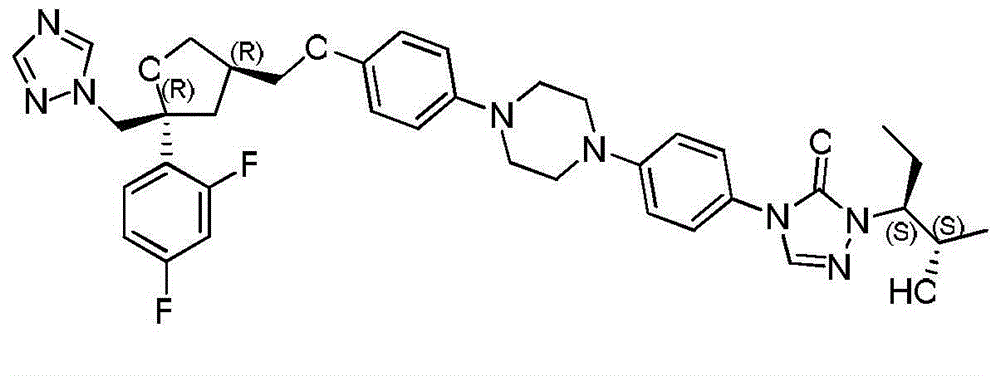
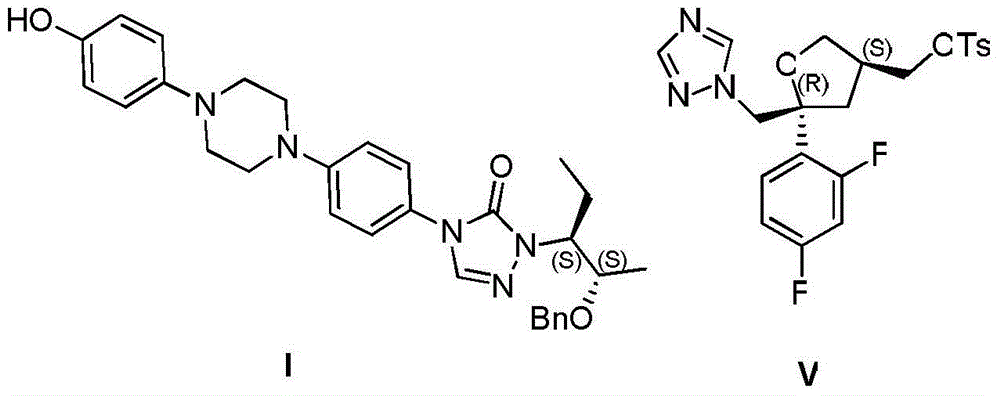
[0031] The preparation of compound I:
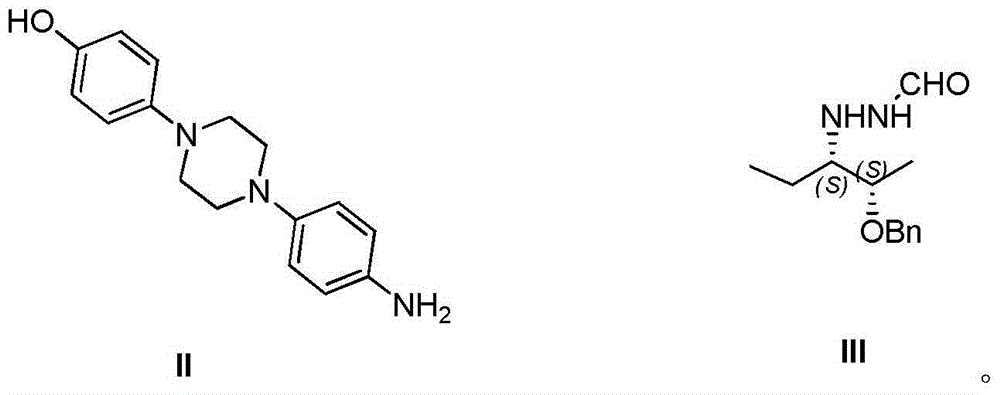
[0032] Add 1-(4-aminophenyl)-4-(4-hydroxyphenyl)piperazine (compound II) (0.5 g, 1.8 mmol) and DMF (10 mL) into a 50 mL four-necked flask, and stir to dissolve. Slowly add phenyl chloroformate (0.28mL, 2.2mmol) dropwise at 0-10°C, continue to react at room temperature for 1-3h after the dropwise addition, after the reaction is monitored by HPLC, add 2-[(1S,2S )-1-ethyl-2-benzyloxypropyl]hydrazine carboxaldehyde (compound III) (0.6g, 2.5mmol) and triethylamine (0.36mL, 2.5mmol), heated to 90-100°C for 15-24h , HPLC monitoring after the end of the reaction, stop heating, the reaction solution down to room temperature.
[0033] Isolation of Compound I:
[0034] At room temperature, the reaction solution was slowly poured into water (100mL), a large amount of solids precipitated, continued to stir for 1h, filtered, the filter cake was washed with water (10mL*3), the solid was dissolved in THF (10mL) after draining, and activated carbon was...
Embodiment 2
[0038] Preparation of compound I:
[0039] Add 1-(4-aminophenyl)-4-(4-hydroxyphenyl)piperazine (compound II) (5.0 g, 18 mmol) and DMF (100 mL) into a 250 mL four-necked flask, and stir to dissolve. Slowly add phenyl chloroformate (2.4mL, 18.9mmol) dropwise at 10-20°C, and continue to react at room temperature for 2-5h after the dropwise addition is completed. After the reaction is monitored by HPLC, add 2-[(1S, 2S )-1-ethyl-2-benzyloxypropyl]hydrazine carboxaldehyde (compound III) (5.1g, 21.6mmol) and triethylamine (3.1mL, 21.6mmol), heated to 100 ~ 110 ℃ reflux reaction for 12 ~ After 15 hours, after the reaction was monitored by HPLC, the heating was stopped, and the reaction solution was cooled to room temperature.
[0040] Isolation of Compound I:
[0041] At room temperature, the reaction solution was transferred to an Erlenmeyer flask, and saturated sodium chloride solution (200mL) and dichloromethane (200mL) were added, stirred vigorously for 30min and left to separat...
Embodiment 3
[0045] Preparation of compound I:
[0046] Add 1-(4-aminophenyl)-4-(4-hydroxyphenyl)piperazine (compound II) (5.0 g, 18 mmol) and DMSO (100 mL) into a 250 mL four-necked flask, and stir to dissolve. 25~30℃, slowly add phenyl chloroformate (3.5mL, 27mmol) dropwise, continue to react at room temperature for 6~8h after the dropwise addition, after the reaction is monitored by HPLC, add 2-[(1S,2S) -1-Ethyl-2-benzyloxypropyl]hydrazinecarbaldehyde (compound III) (6.8g, 28.8mmol) and triethylamine (4.1mL, 28.8mmol), heated to 110-120°C for 5-8h , HPLC monitoring after the end of the reaction, stop heating, the reaction solution down to room temperature.
[0047] Isolation of Compound I:
[0048] At room temperature, the reaction solution was transferred to an Erlenmeyer flask, and saturated sodium chloride solution (200mL) and ethyl acetate (200mL) were added, stirred vigorously for 30min and left to separate the liquids, and the aqueous phase was extracted with ethyl acetate (200m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com