Compounds of '3-(5-sustituted oxy-2,4-dinitro-phenyl)-2-oxo-propionic acid ester', process and applications thereof
A compound and alkynyl technology, applied in the preparation of nitro compounds, organic chemistry, etc., can solve problems such as tedious work process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
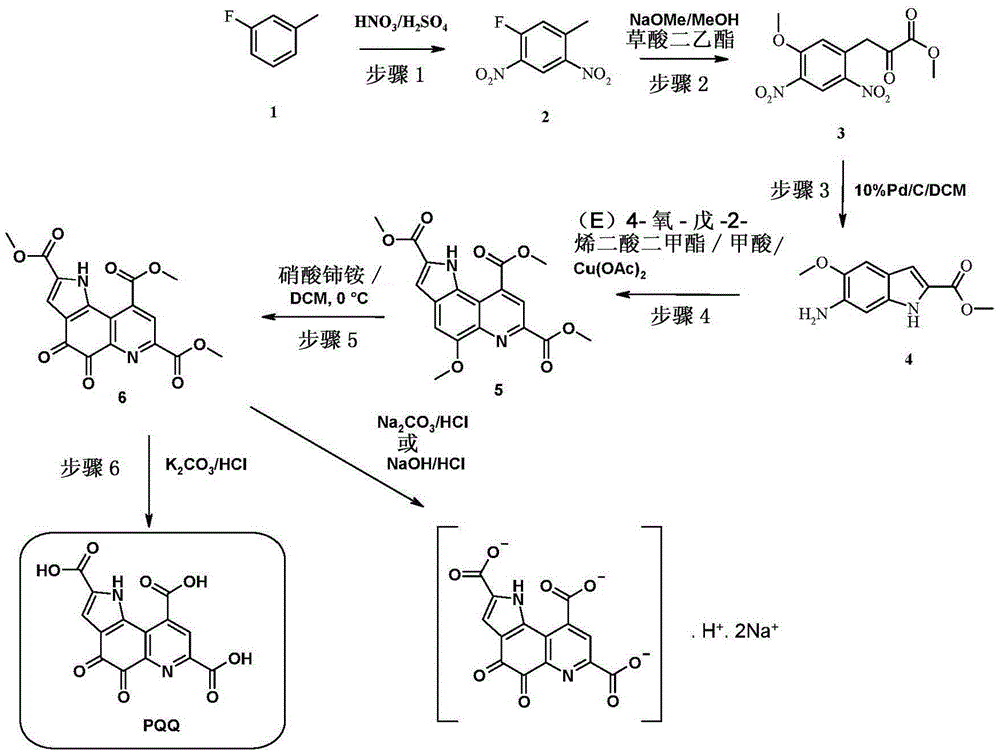
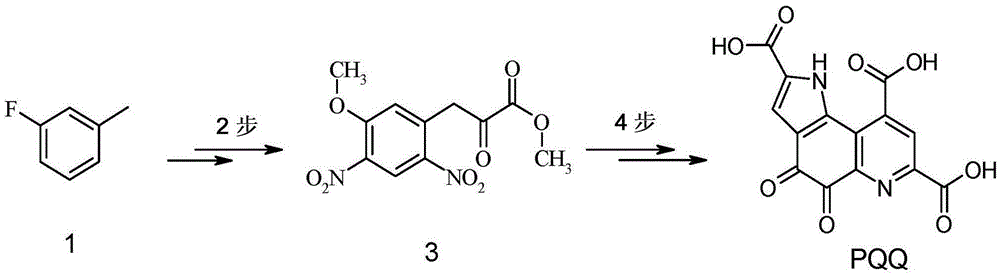
[0185] Example 1: Preparation of 1-fluoro-5-methyl-2,4-dinitro-benzene [formula III, wherein R3 is F]
[0186]
[0187] 67% nitric acid (approximately 250 mL, 5.6 moles) was charged at a temperature of approximately 25-30 °C to a three-neck round bottom flask equipped with a mechanical stirrer. Thereafter concentrated sulfuric acid (about 360 mL, 6.75 mol) was added dropwise at about 0°C over a period of about 30 minutes. 1-Fluoro-3-methyl-benzene [formula II, wherein R3 is F] (about 100 mL, 0.9 mol) was added dropwise to the obtained Nitrate mixture.
[0188] The reaction mixture obtained was stirred at a temperature in the range of about 20-25° C. during about 30 minutes. The completion of the reaction was monitored by thin layer chromatography (TLC) (10% ethyl acetate in petroleum ether as solvent system). After this time, the reaction mixture was poured onto crushed ice (about 3.0 L) to precipitate the product. The precipitated product was filtered and dried under...
Embodiment 2A
[0192] Example 2A: 3-(5-methoxy-2,4-dinitro-phenyl)-2-oxo-propionic acid methyl ester [formula I, wherein, R1 is-CH 3 and R2 is -CH 3 ] preparation
[0193]
[0194] To a suspension of 1-fluoro-5-methyl-2,4-dinitro-benzene (ca. about 108 g, 1.9 moles) and diethyl oxalate (136 mL, 1.0 moles). The obtained orange suspension was brought slowly to a temperature of about 15-20° C. in about 2 hours and stirred continuously at a temperature of about 15-20° C. during a period of about 16 hours. The reaction mixture was quenched with 1.5N HCl (about 200 mL), and the resulting precipitated solid was filtered and dried under house vacuum to obtain the product [3-(5-methoxy-2,4-dinitro- Phenyl)-2-oxo-propionic acid methyl ester] (ca. 125 g, 85%).
[0195] 1 HNMR (CDCl 3 , 300MHz): 3.98(s, 3H), 4.11(s, 3H), 4.65(s, 2H), 7.00(s, 1H), 8.84(s, 1H);
[0196] LC-MS (ESI): 297.0 (M-H);
[0197] HPLC purity: 99.12%
[0198] Melting range: 142.7°C-143.2°C
[0199] IR (KBr,...
Embodiment 2B
[0220] Example 2B: Methyl 3-(5-methoxy-2,4-dinitro-phenyl)-2-oxo-propionate [sodium salt of formula I, which where R1 is -CH 3 and R2 is -CH 3 ] preparation
[0221]
[0222] Sodium salt of formula I
[0223] To a suspension of 1-fluoro-5-methyl-2,4-dinitro-benzene (ca. 4.0 g, 16.0 mmol) in methanol (ca. 50 mL) was added sodium methoxide at a temperature of about 0 °C (about 4.3g, 76mmol) and diethyl oxalate (about 5.4mL, 37mmol). The obtained orange suspension was slowly brought to a temperature of about 15-20° C. within 2 hours and stirred continuously at a temperature of about 15-20° C. during a period of about 16 hours. The completion of the reaction was monitored by TLC. The obtained precipitated solid was filtered and dried under house vacuum to obtain the product sodium [2-hydroxy-3-(5-methoxy-2,4-dinitro-phenyl)-methylacrylate as a brown solid salt] (about 5.0 g, 86%).
[0224] 1 HNMR (DMSO-d 6 ,400MHz): 3.66(s, 3H), 3.87(s, 3H), 6.87(s, 1H), 8.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com