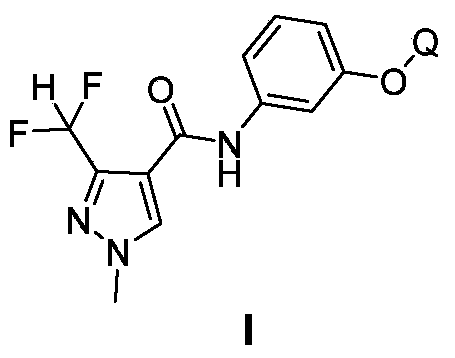
Pyrazole amide compounds and applications thereof
A kind of technology of pyrazole amide and compound, applied in pyrazole amide compound and its application field, can solve the problem that structure pyrazole amide compound has not been reported and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] The preparation of embodiment 1 compound 42:
[0106]
[0107] Add 3-(5-trifluoromethylpyridine-2-oxyl group) aniline (500 mg, 2.0 mmoles, synthetic method reference pesticide, 2007,45(5):307-309), triethylamine ( 200 mg, 2.0 mmol) and 10 ml of dichloromethane, add dropwise a solution of 1-methyl-3-difluoromethylpyrazole-4-carbonyl chloride (360 mg, 2.0 mmol) in dichloromethane under stirring at room temperature 10 ml. After dropping, stir overnight at room temperature. The reaction solution was poured into 30 ml of water, and the organic layer was taken. The organic layer was washed with saturated aqueous sodium bicarbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography (eluent: ethyl acetate: petroleum ether = 1:1) to obtain 740 mg of compound 42, a yellow solid, melting point 122-124°C.
Embodiment 2
[0108] The preparation of embodiment 2 compound 53:
[0109]
[0110] Add N-(2-(3-aminophenoxy)acetyl)morpholine (370 mg, 1.58 mmol, synthetic method reference Pesticide, 2007,45(5):307-309), triethylamine in the reaction flask (160 mg, 1.58 mmol) and 20 ml of acetonitrile, under stirring in an ice bath, 5 ml of acetonitrile solution of 1-methyl-3-difluoromethylpyrazole-4-formyl chloride (280 mg, 1.42 mmol) was added dropwise . After dropping, reflux, and the reaction was completed after 2 hours. The reaction solution was poured into 30 ml of water, and extracted with 50 ml of ethyl acetate. The organic layer was taken, washed with saturated aqueous sodium bicarbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography (eluent: ethyl acetate: petroleum ether = 3:1) to obtain 100 mg of compound 53 as a yellow solid, melting point 145-146°C.
[0111] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com