Three-dimensional phase-change material based on graphene and preparing method of three-dimensional phase-change material
A phase change material, graphene technology, applied in heat exchange materials, chemical instruments and methods, sustainable manufacturing/processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) The graphene oxide aqueous solution with a concentration of 60 mg / mL was placed in liquid nitrogen and frozen for 3 min, 5 min, 15 min, 30 min, and 35 min, respectively. The frozen graphene oxide gels (a, b, c, d, e) were observed under a scanning electron microscope. The graphene oxide gel a had more sheets and fewer holes; the graphene oxide gel b, c, d The pores are uniform, the degree of density increases sequentially, and the structure of graphene oxide gel e is destroyed.
[0020] (2) Heating the frozen graphene oxide gel to 800°C at a rate of 1°C / min in an inert gas atmosphere, and keeping it warm for 0.5h;
[0021] (3) Raise the temperature to 1300°C at a rate of 3°C / min in an inert gas atmosphere and keep it warm for 0.5h;
[0022] (4) In an inert gas atmosphere, the temperature was raised to 3000°C at a rate of 8°C / min, and kept for 0.5h to obtain graphene airgel;
[0023] (5) Soak the graphene airgel in a dichloromethane solution of paraffin wax with a ...
Embodiment 2
[0025] (1) The graphene oxide aqueous solution with a concentration of 1 mg / mL was placed in liquid nitrogen for 25 min.
[0026] (2) Heating the frozen graphene oxide gel to 800°C at a rate of 1°C / min in an inert gas atmosphere, and keeping it warm for 0.5h;
[0027] (3) Raise the temperature to 1300°C at a rate of 3°C / min in an inert gas atmosphere and keep it warm for 0.5h;
[0028] (4) In an inert gas atmosphere, the temperature was raised to 3000°C at a rate of 8°C / min, and kept for 0.5h to obtain graphene airgel;
[0029] (5) Soak the airgel obtained in step 4 in a paraffin-dichloromethane solution with a concentration of 10g / ml for 5h, and then dry it in a vacuum oven at 30°C to obtain a three-dimensional phase change material based on graphene, filled with paraffin The amount is 70%. Through the differential scanning calorimetry (DSC) test, the melting phase change enthalpy of the graphene-based three-dimensional phase change material is 126.3J / g, and the solidificat...
Embodiment 4
[0031] (1) Place the graphene oxide aqueous solution with a concentration of 100 mg / mL in liquid nitrogen for 25 min.
[0032] (2) The graphene oxide gel after the freezing treatment is heat-treated with the heat treatment methods shown in Table 1 to Table 3 under an inert gas atmosphere,
[0033] (3) Soak the airgel after the heat treatment in step 3 in a paraffin-dichloromethane solution with a concentration of 10 g / ml for 3 hours, and then dry it in a vacuum oven at 30° C. to obtain a graphene-based three-dimensional phase change material.
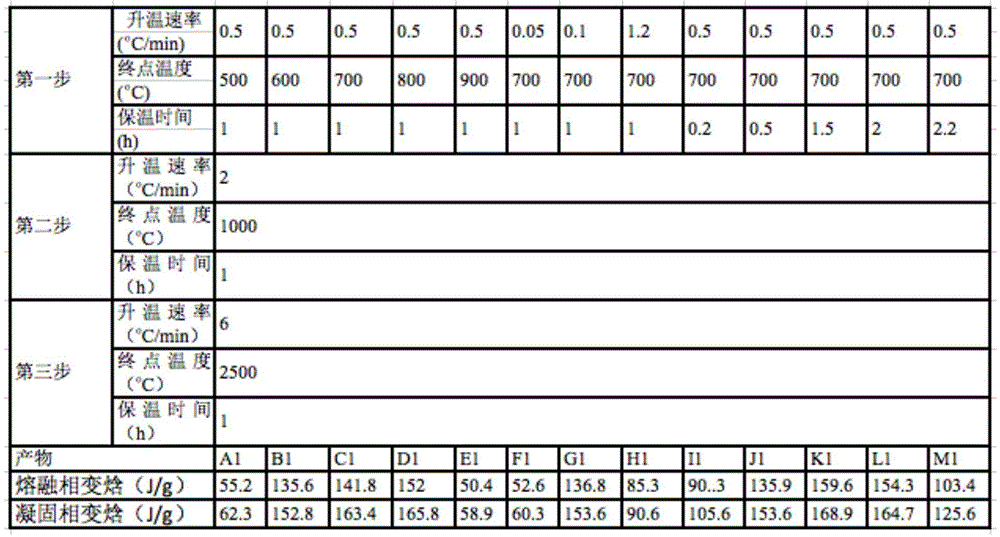
[0034] Table 1
[0035]
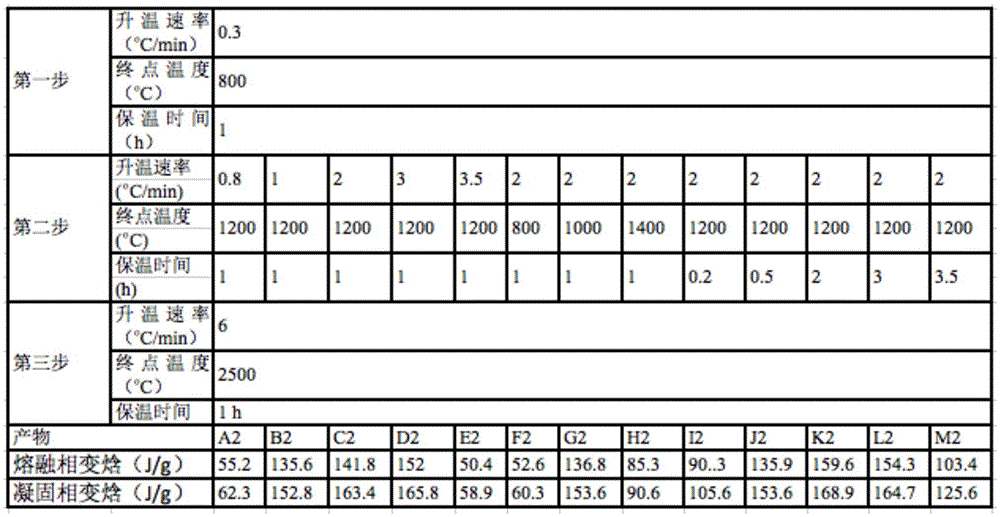
[0036] Table 2
[0037]
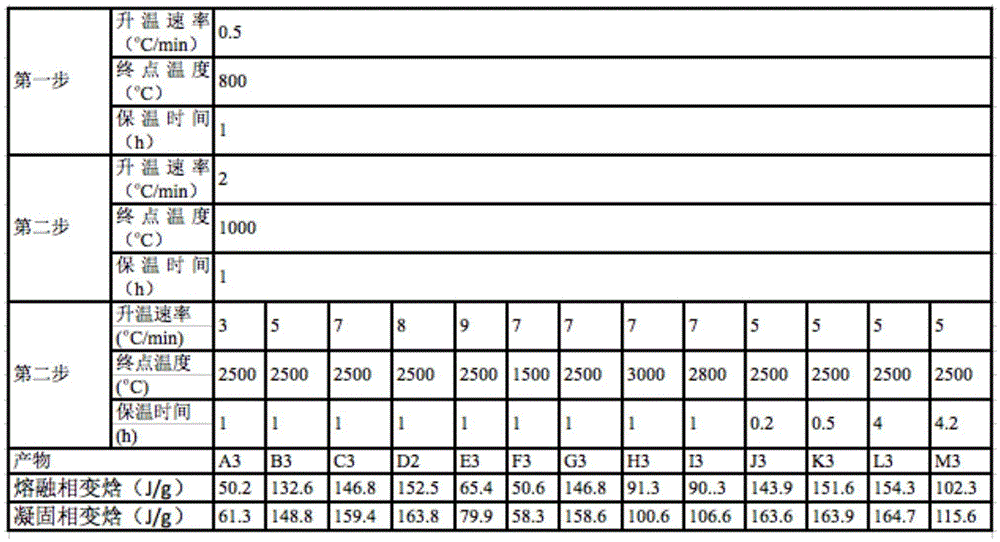
[0038] table 3
[0039]
[0040] It can be seen from Tables 1 to 3 that the performance of this material is mainly determined by two aspects. One is the repair of the graphene oxide sheet structure inside the material, that is, the loss of functional groups and the repair of the carbon conjugated structure at high temperature. Second, the continuity of the three-dimensional...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com