A kind of synthetic method of positive electrode material of lithium ion battery
A lithium-ion battery and cathode material technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of slow nucleation rate and larger primary particle size, improve production efficiency, facilitate mass production, The effect of reducing particle growth time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 10L reactor, control the nitrogen ventilation rate to 200mL / min to remove oxygen in and above the solution, stir at 1000rpm and control the temperature in the kettle to 50°C.
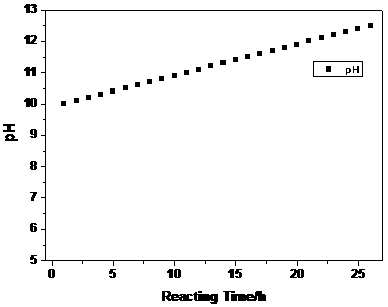
[0023] Configure a 3M metal ion aqueous solution, in which the molar ratio of nickel sulfate: cobalt sulfate: manganese sulfate is 5:2:3, and then enter the reaction kettle at a speed of 10mL / min, while the aqueous sodium hydroxide solution is figure 1 The set pH value is automatically added to the kettle by the automatic equipment controlled by the program. The pH value at the beginning of the reaction is set to 10, and the pH value is controlled to increase linearly. The pH value change rate V is , the reaction time is 26h, and at the same time, the ammonia solution is continuously pumped into the reaction kettle to maintain the required constant ammonia concentration in the kettle.
[0024] The synthesized precursor was sintered in two steps to prepare the desired positive electrode mate...
Embodiment 2
[0026] In a 10L reactor, control the nitrogen ventilation rate to 200mL / min to remove oxygen in and above the solution, stir at 1000rpm and control the temperature in the kettle to 50°C.
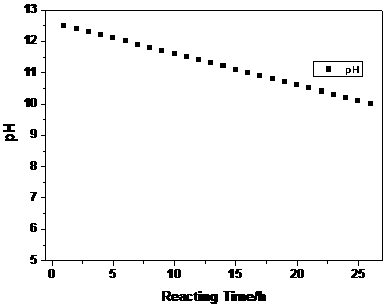
[0027] Configure a 3M metal ion aqueous solution, in which the molar ratio of nickel sulfate: cobalt sulfate: manganese sulfate is 5:2:3, and then enter the reaction kettle at a speed of 10mL / min, while the aqueous sodium hydroxide solution is figure 2 The set pH value is automatically added to the kettle by the automatic equipment controlled by the program. The pH value at the beginning of the reaction is set to 12.5, and the pH value is controlled to decrease linearly. The pH value change rate V is , the reaction time is 26h, and at the same time, the ammonia solution is continuously pumped into the reaction kettle to maintain the required constant ammonia concentration in the kettle.
[0028] The synthesized precursor was sintered in two steps to prepare the desired positive electrode m...
Embodiment 3
[0030] In a 10L reactor, control the nitrogen ventilation rate to 200mL / min to remove oxygen in and above the solution, stir at 1000rpm and control the temperature in the kettle to 50°C.
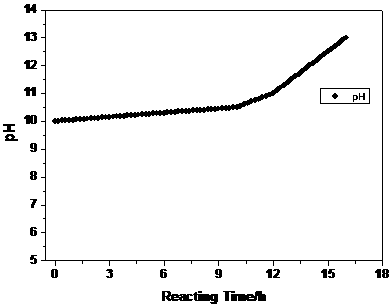
[0031] Configure a 3M metal ion aqueous solution, in which the molar ratio of nickel sulfate: cobalt sulfate: manganese sulfate is 6:2:2, and then enter the reaction kettle at a speed of 10mL / min, while the aqueous sodium hydroxide solution is image 3 The set pH value is automatically added into the kettle by the automatic equipment controlled by the program. The pH value at the beginning of the reaction is set to 10, and the pH value is controlled to increase linearly in multiple stages. The pH change rate of the first stage is V 1 for , the reaction time is 10h; the second stage increases linearly, and the pH change rate V 2 for , the reaction time is 2h; in the third stage, the pH value increases linearly, and the pH change rate V 3 for , The reaction time is 4h. At the same time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com