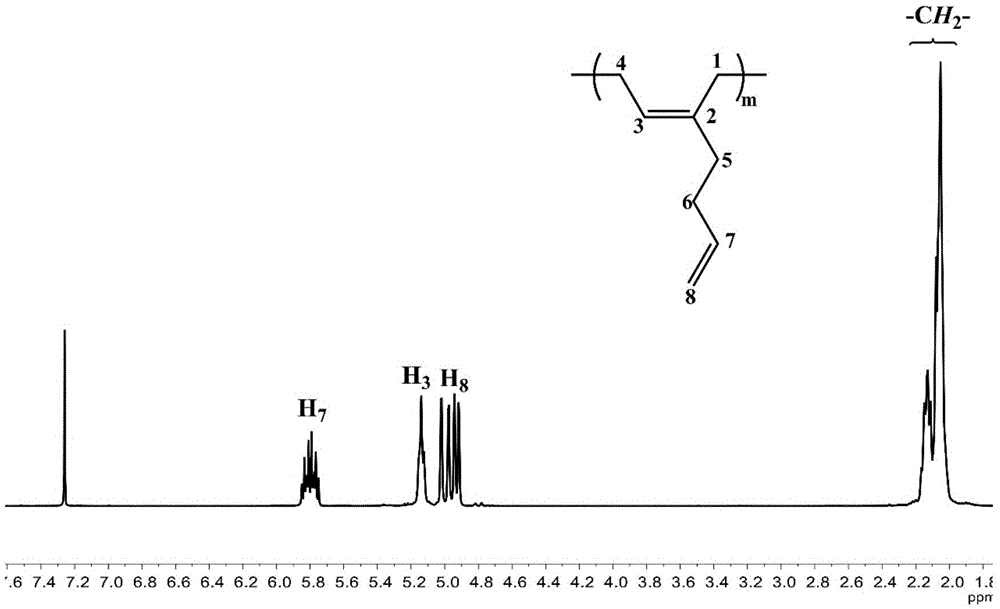
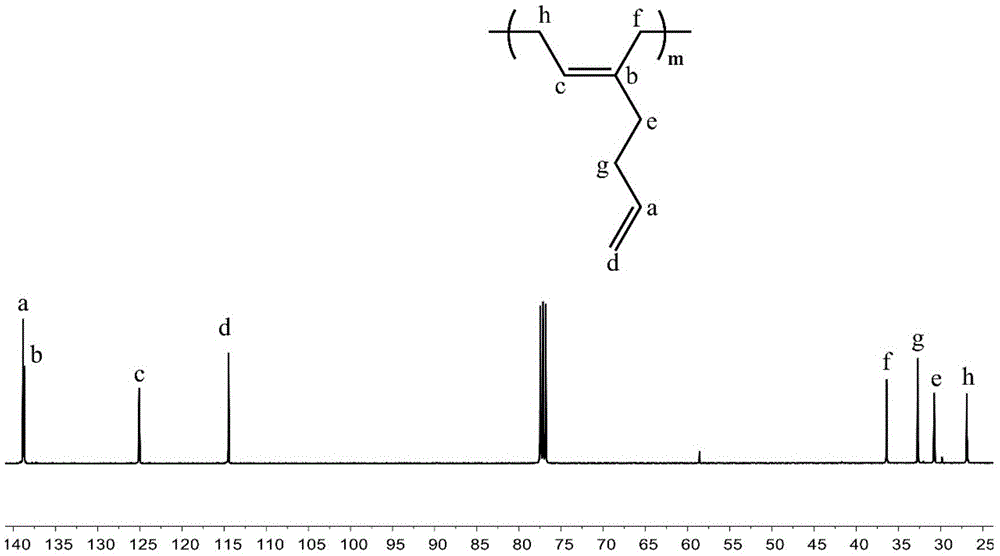
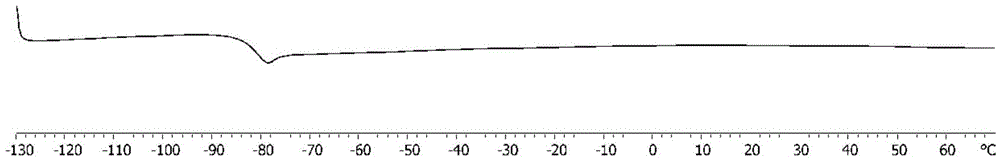
High CIS-1,4 conjugated diene polymer with double bonds on side arm and preparation method thereof as well as high CIS-1,4 conjugated diene polymer with functional groups on side arm and preparation method thereof
A diolefin and polymer technology, which is applied in the field of high cis-1,4 conjugated diolefin polymers with functional groups in side arms and the preparation method, can solve the problems of inability to obtain high stereoregularity conjugated diolefin polymers and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] A method for preparing a side arm with a double bond high cis-1,4 conjugated diene polymer, comprising the following steps:
[0043] Under the action of catalyst and co-catalyst, the side arm with double bond high cis- 1,4 conjugated diene polymer;
[0044]
[0045] where z=0,1,2,3 or 4.
[0046] The catalyst is a tridentate carbazole-based rare earth complex shown in formula (III), and its structural formula is as follows:
[0047]
[0048] Among them, R 2 is hydrogen, methyl, ethyl, isopropyl, tert-butyl, methoxy, phenyl or benzyl; R 3 is methyl, ethyl, propyl, isopropyl, butyl, tert-butyl, phenyl, benzyl or cyclohexyl; Ln is rare earth metal Y, Lu, Sc, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Ho, Er, Tm or Yb.
[0049] The cocatalyst is organoboron salt [Ph 3 C][B(C 6 f 5 ) 4 ], [PhNMe 2 H][B(C 6 f 5 ) 4 ], [Ph 3 C][BPh 4 ], [PhNMe 2 H][BPh 4 ] or B(C 6 f 5 ) 3 .
[0050] During the preparation of the high cis-1,4 conjugated diene polymers with doub...
Embodiment 1
[0071] Under the protection of nitrogen, add 2.5mL of anhydrous toluene and 2.5mmol of 2-methylene-1,6-heptadiene to the dry polymerization reactor successively, then put it into a constant temperature device at 25°C, and add 10μmol of catalyst Tridentate carbazolyl rare earth complexes (R 2 is tert-butyl, R 3 is phenyl, Ln metal Y) and 10 μmol cocatalyst organoboron salt ([Ph 3 C][B(C 6 f 5 ) 4 ])) to initiate the reaction, and after 5 minutes of reaction, the reaction solution was poured into an ethanol solution containing 10% (V / V) hydrochloric acid, and a white polymer was precipitated. The filtered product was washed with ethanol for 3 times, and then dried in a vacuum oven at 50° C. for 24 to 36 hours to obtain 0.27 g of a high cis-1,4 conjugated diene homopolymer with double bonds in side arms. The number average molecular weight (M n ) is 32.9kg / mol, molecular weight distribution (M w / M n ) is 1.12, and the polymer glass transition temperature (T g ) is -82.3...
Embodiment 2
[0073] Change the added anhydrous toluene and 2-methylene-1,6-heptadiene in Example 1 to 10mL and 10mL respectively, change the reaction time to 60min, and keep other conditions unchanged, and finally obtain the side arm with a double bond High cis-1,4 conjugated diene homopolymer 1.08g. The number average molecular weight (M n ) is 121kg / mol, molecular weight distribution (M w / M n ) is 1.08, and the polymer glass transition temperature (T g ) is -81.9°C. pass 1 H and 13 CNMR spectrum analysis calculates the tacticity of the polymer to be cis-1,4 content of 98.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com