Anti-foot-and-mouth disease vaccine composition and preparation method and application thereof
A vaccine composition and composition technology, applied in the direction of biochemical equipment and methods, methods based on microorganisms, medical preparations containing active ingredients, etc., can solve the problems of short validity period, incompletely killed or fully attenuated viruses, and pollution And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Protein expression of foot-and-mouth disease having sequences SEQ ID NO.1, 3, 5, 7, 9, 11, 13, 15, 17
[0051] 1. Preparation of foot-and-mouth disease gene fragments used as templates
[0052] The O-type foot-and-mouth disease VP0, VP3, VP1 gene shown in the sequence SEQIDNO.1, 3, 5, the Asian type 1 foot-and-mouth disease VP0, VP3, VP1 gene shown in the sequence SEQIDNO. The full-length genes of type A FMD VP0, VP3, and VP1 shown in 15 and 17 were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. The full lengths of the synthesized gene fragments are 909bp, 660bp, 639bp, 909bp, 657bp, 627bp, 909bp, 663bp, 636bp, respectively. The foot-and-mouth disease gene template of the present invention is prepared on the basis of the artificially synthesized foot-and-mouth disease gene fragment.
[0053] 2. Construction of foot-and-mouth disease gene expression vector
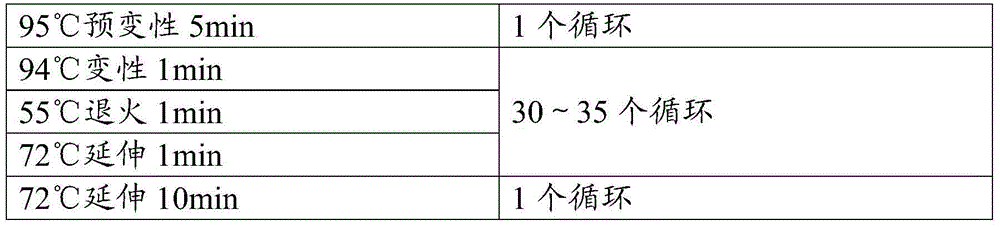
[0054] FMD gene template synthesized in the previous step. Primers were designed respectively (see ...
Embodiment 2
[0068] Preparation of vaccine composition against foot-and-mouth disease
[0069] Get the O-type foot-and-mouth disease virus-like particles prepared in Example 1, the Asian type I foot-and-mouth disease virus-like particles and the A-type foot-and-mouth disease virus-like particles, and slowly add them to the adjuvant, and the process of adding is constantly stirring with a rotating speed of 800rpm emulsifier for 12min, mixing Evenly and stored at 4°C, it becomes the vaccine composition against foot-and-mouth disease. See Table 3 for specific ratios. The adjuvants suitable for the present invention may be adjuvants known to those skilled in the art. In this example, the adjuvant ISA206 (Seppic, France) was selected.
[0070] The composition ratio of the vaccine composition against foot-and-mouth disease of table 3
[0071]
Embodiment 3
[0073] Immunogenicity test of vaccine composition against foot-and-mouth disease
[0074] 1. Immunization procedure
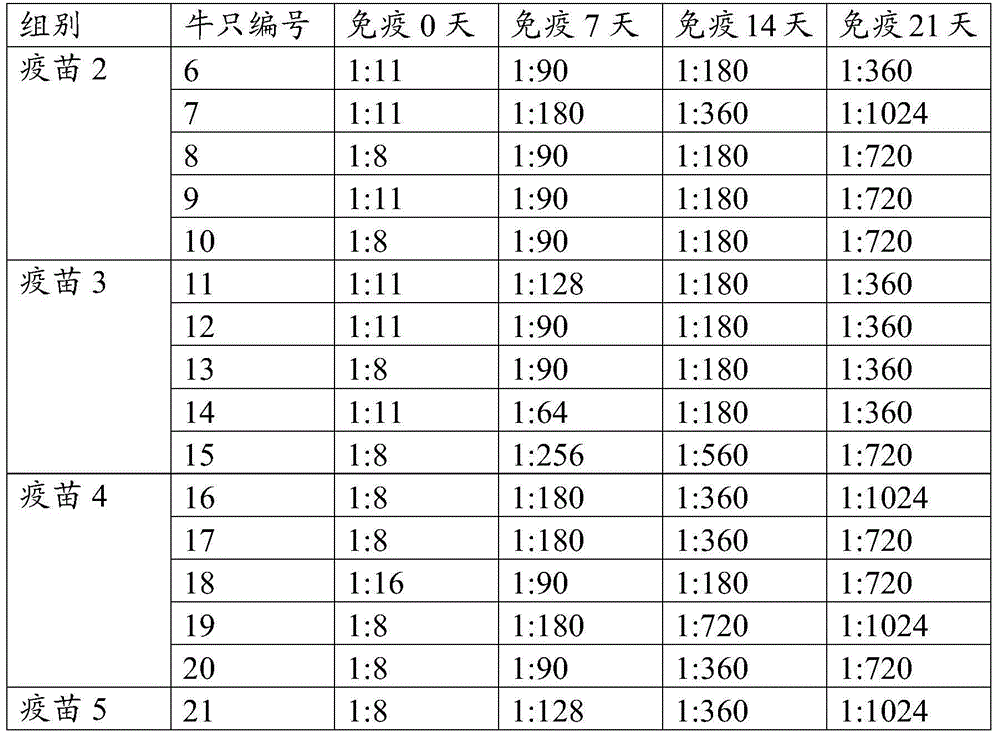
[0075] 30 6-month-old healthy cattle that were negative for the above three antibodies were screened using the FMD O-type antibody ELISA detection kit, the Asian I-type ELISA antibody detection kit and the A-type antibody ELISA detection kit, and were randomly divided into 6 groups. 5 heads per group. Groups 1-5 are vaccine 1, vaccine 2, vaccine 3, vaccine 4, and vaccine 5 immunization groups prepared in Example 2 of the present invention, respectively, and group 6 is a PBS control group. The way of immunization in the immunization group was intramuscular injection of 1 ml in the neck, and the control group was immunized with the same amount of PBS. Blood was collected from each cow before vaccine immunization and every week after immunization until 21 days after immunization.
[0076] 2. Detection of antibody level
[0077] The collected sera were tested f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com