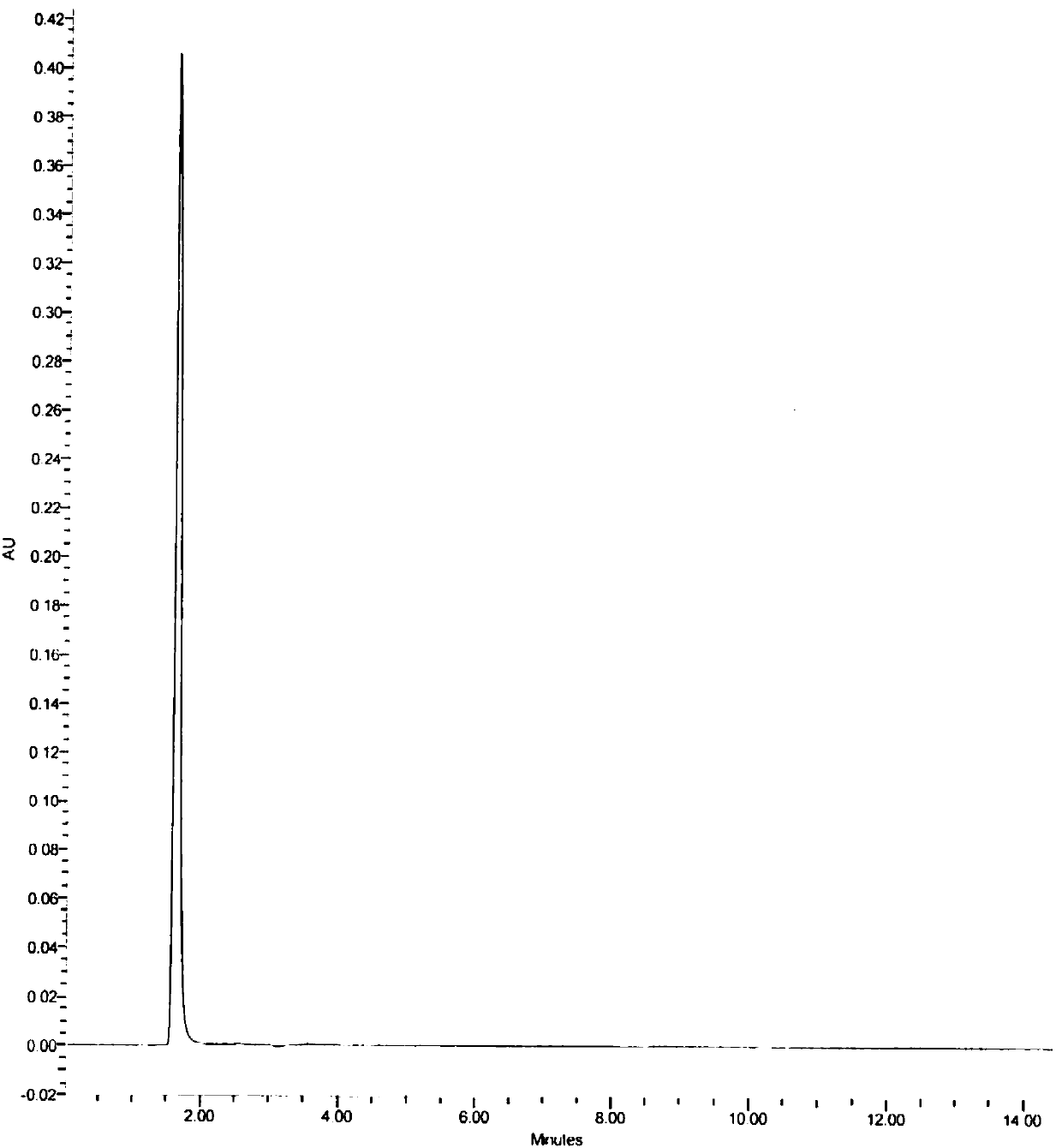
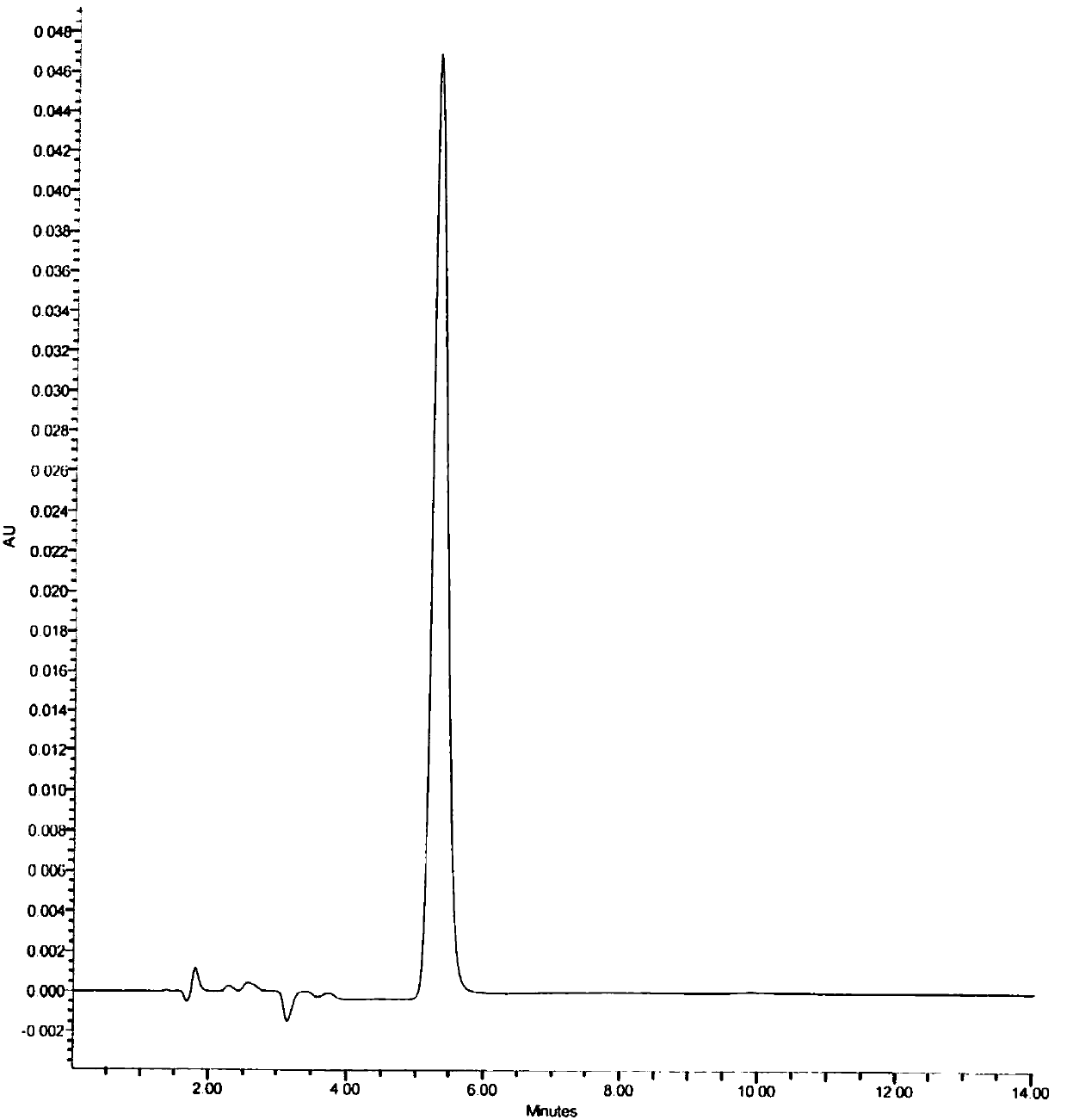
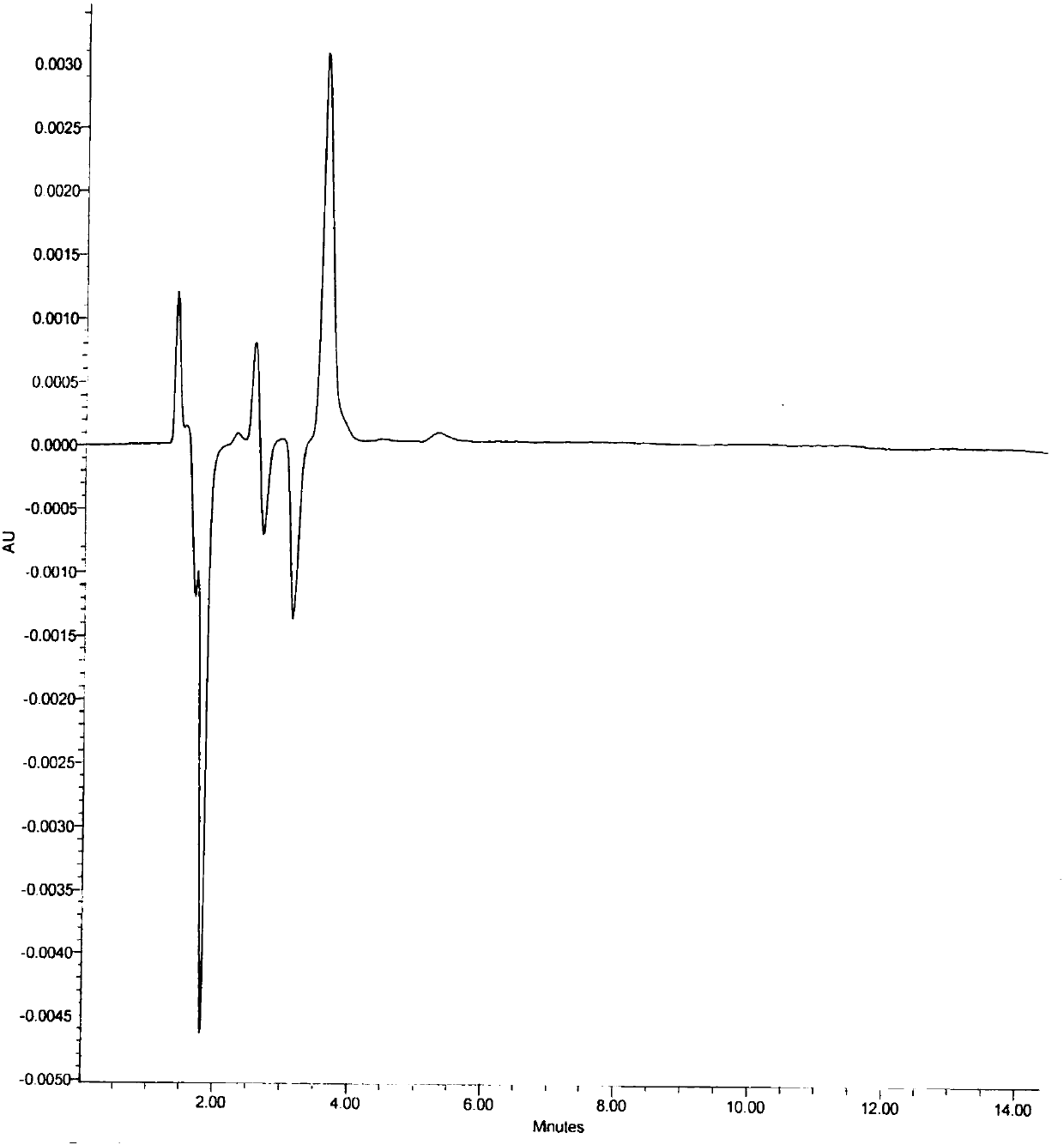
A kind of assay method of stannous content in medicine
A determination method and technology for medicines, which are applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of inaccurate determination of stannous content, cumbersome steps and large errors, and achieve extended retention time, simple steps, and improved accurate determination. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The assay method of stannous content in the medicine described in this implementation comprises the steps:
[0069] (1) preparation of need testing solution: get need testing, add mobile phase A and mobile phase B solution solution mixed by elution ratio to dissolve and dilute, obtain the sample solution that stannous amount concentration is 0.01mg / mL, i.e. have to;
[0070] (2) Preparation of reference substance solution: get tin protochloride (SnCl 2 2H 2 (2) 100mg, accurately weighed, placed in a 10mL measuring bottle, added 0.05mol / L dilute hydrochloric acid to dissolve and diluted to the scale, to obtain a stannous chloride solution, and accurately measure 0.05mL of the stannous chloride solution and place it in 10mL In the measuring bottle, add the mobile phase A and mobile phase B solution mixed according to the elution ratio and dilute to the mark to obtain;
[0071] Chromatographic conditions and system suitability test: According to high performance liquid ...
Embodiment 2
[0074] The assay method of stannous content in the medicine described in this implementation comprises the steps:
[0075] (1) Preparation of need testing solution: get need testing, add mobile phase A and mobile phase B solution mixed by elution ratio to dissolve and dilute, obtain the sample solution that stannous amount concentration is 0.5mg / mL, obtain final product ;
[0076] (2) Preparation of reference substance solution: get tin protochloride (SnCl 2 2H 2(2) 100mg, accurately weighed, placed in a 10mL measuring bottle, added 0.03mol / L dilute hydrochloric acid to dissolve and diluted to the mark, to obtain a stannous chloride solution, and accurately measure 0.15mL of the stannous chloride solution and place it in 10mL In the measuring bottle, add the mobile phase A and mobile phase B solution mixed according to the elution ratio and dilute to the mark to obtain;
[0077] Chromatographic conditions and system suitability test: According to high performance liquid chr...
Embodiment 3
[0080] The assay method of stannous content in the medicine described in this implementation comprises the steps:
[0081] (1) preparation of need testing solution: get need testing, add mobile phase A and mobile phase B solution mixed by elution ratio to dissolve and dilute, obtain the sample solution that stannous amount concentration is 0.01mg / mL, obtain final product ;
[0082] (2) Preparation of reference substance solution: get tin protochloride (SnCl 2 2H 2 (2) 100mg, accurately weighed, placed in a 10mL measuring bottle, added 0.05mol / L dilute hydrochloric acid to dissolve and diluted to the mark, to obtain a tin protochloride solution, accurately measure 0.1 mL of the stannous chloride solution and place it in 10 mL In the measuring bottle, add the mobile phase A and mobile phase B solution mixed according to the elution ratio and dilute to the mark to obtain;
[0083] Chromatographic conditions and system suitability test: According to high performance liquid chro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com