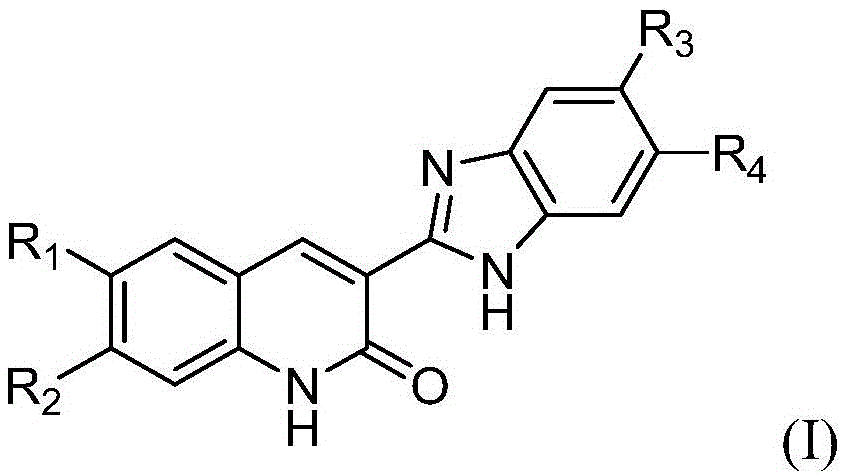
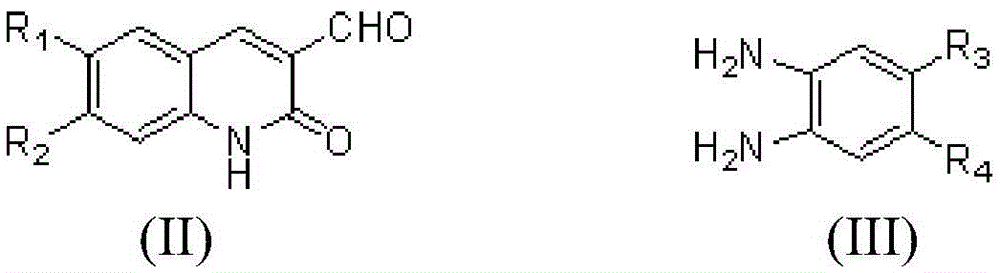
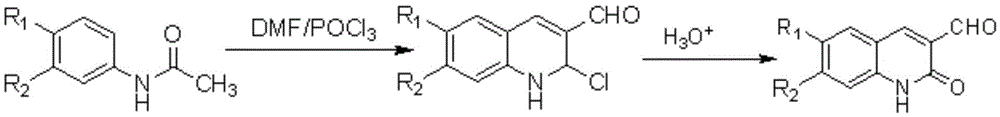
3-benzimidazole-2(1H)-quinolinone derivative and preparation method and application thereof
A reaction and compound technology, applied in the field of medicine, to achieve good medicinal value, stable quality, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] 1. Preparation of 1a: Mix 3.5mL of DMF and 17mL of POCl 3 After mixing and stirring evenly, add 2.03g of acetanilide, raise the temperature to 90°C, heat to reflux for 16h, pour into a large amount of ice water after cooling, and filter to obtain a yellow powder. The obtained yellow powder was mixed with 200 mL of 70% acetic acid solution, refluxed at 95° C. for 8 h, and cooled to obtain compound 1a as a yellow needle-like solid, 91%.
[0034] Compound 1a: 1 HNMR (500MHz, DMSO-d 6 )δ: 12.22(s,1H,NH),10.23(s,1H,CHO),8.49(s,1H,C=CH),7.91(d,J=7.9Hz,1H,Ar–H),7.65( t,J=7.8Hz,1H,Ar–H),7.35(d,J=8.3Hz,1H,Ar–H),7.25(t,J=8.0Hz,1H,Ar–H); 13 CNMR (126MHz, DMSO-d 6 )δ: 190.24, 161.90, 142.92, 141.60, 134.16, 131.38, 126.07, 123.14, 118.60, 115.89. MSm / z: 174[M+H] + .
[0035] 2. Preparation of 2a: Referring to the preparation steps of compound 1a, p-methylacetanilide was used instead of acetanilide to obtain compound 2a with a yield of 87%. The crystal structure is shown in th...
Embodiment 1
[0041] Example 1: 3-(5,6-dimethyl-1H-benzimidazol-2-yl)-2(1H)-quinolinone (compound 1a 1 ) preparation
[0042] Weigh 0.1g (0.58mmol) of compound 1a, 0.09g (0.64mmol) of 4,5-dimethyl-1,2-phthalate, and 6mL of anhydrous methanol and place them in a pressure-resistant test tube (sealed tube). Under the conditions of 85°C, ultrasonic frequency of 40kHz, and ultrasonic power of 540W, the reaction was complete (TLC tracking detection, about 1h). After cooling, it was filtered with suction and washed with absolute ethanol to obtain compound 1a 1 0.148g, yield 88.3%.
[0043] Compound 1a 1 : Yields88.3%, 1 HNMR (500MHz, DMSO-d 6 )δ12.42(s,2H,NH),9.05(s,1H,H-Ar),7.94(d,J=7.2Hz,1H,H-Ar),7.60(s,1H,H-Ar), 7.43(s, 3H, H-Ar), 7.28(d, J=7.5Hz, 1H, H-Ar), 2.30(s, 6H, 2CH 3 -); 13 CNMR (126MHz, DMSO-d 6 )δ161.28,147.31,138.99,138.83,131.80,129.38,123.07,120.75,119.70,115.68,20.59.MSm / z:290[M+H] + .
[0044] Therefore, it can be determined that the above compound 1a 1 It is 3-(5,6-d...
Embodiment 2
[0046] Example 2: 3-(5,6-dichloro-1H-benzimidazol-2-yl)-2(1H)-quinolinone (compound 1a 2 ) preparation
[0047] Weigh 0.1g (0.58mmol) of compound 1a, 0.11g (0.64mmol) of 4,5-dichloro-1,2-o-phenylenediamine, and 6mL of anhydrous methanol into a pressure-resistant test tube (sealed tube), at 80 Stir the reaction at ℃ until complete (TLC tracking detection, about 6h), filter with suction after cooling, and wash with absolute ethanol to obtain compound 1a 2 0.136 g, yield 71.4%.
[0048] Compound 1a 2 : Yields71.4%, 1 HNMR (500MHz, DMSO-d 6 )δ12.89(s,1H,NH),12.53(s,1H,NH),9.12(s,1H,H-Ar),7.97(d,J=7.1Hz,1H,H-Ar),7.93( s,2H,H-Ar),7.64(s,1H,H-Ar),7.45(s,1H,H-Ar),7.30(d,J=7.5Hz,1H,H-Ar); 13 CNMR (126MHz, DMSO-d 6 )δ161.26, 161.10, 153.43, 150.77, 140.59, 139.42, 132.54, 129.75, 124.91, 123.23, 119.61, 119.48, 115.81, 115.31. MSm / z: 330[M+H] + .
[0049] Therefore, it can be determined that the above compound 1a 2 It is 3-(5,6-dichloro-1H-benzimidazol-2-yl)-2(1H)-quinolinone,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com