Method for constructing cartilage tissues by aid of human urine cells
A cartilage tissue and urine cell technology, applied in the biological field, can solve the problems of limiting the application of bone marrow mesenchymal stem cells, pain at the sampling point, and large trauma.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] [Example 1] A method for reprogramming urine cells into induced pluripotent stem cells
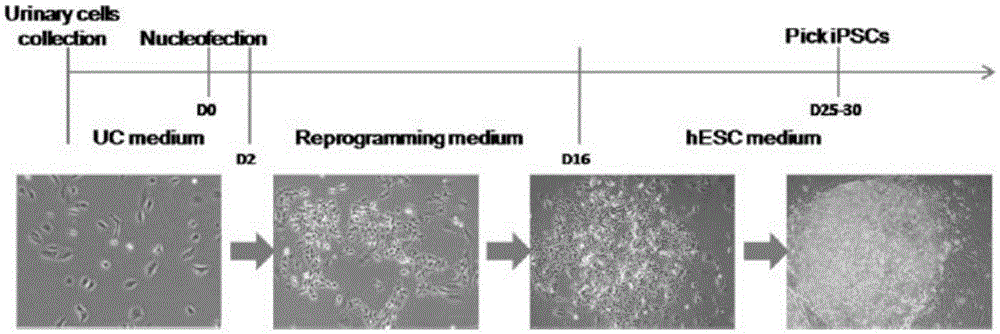
[0064] This embodiment relates to a method for generating iPS cells mediated by non-integrated attachment vectors derived from urine cells, which specifically includes the following steps ( figure 1 ):
[0065] 1) Introduce non-integrated episomal vectors (pEP4EO2SEN2K: 3.0 μg, pEP4EO2SET2K: 3.2 μg, pCEP4-M2L: 2.4 μg) expressing transcriptional regulators OCT4, SOX2, NANOG, KLF4 and LIN28 into 1 million urine cells, and then The cells were divided into three 10cm dishes pre-coated with matrigel and cultured with fibroblast medium;
[0066] 2) On the second day after transfection, the fibroblast medium was replaced with N2B27 conditioned medium (containing MEK inhibitor PD0325901, GSK3b inhibitor CHIR99021, TGF-b / Activin / Nodalreceptor inhibitor A-83-01, ROCK inhibitor HA- 100and human leukemia inhibitory factor (LIF), replace fresh medium every 2 days;
[0067] 3) On the 13th day ...
Embodiment 2
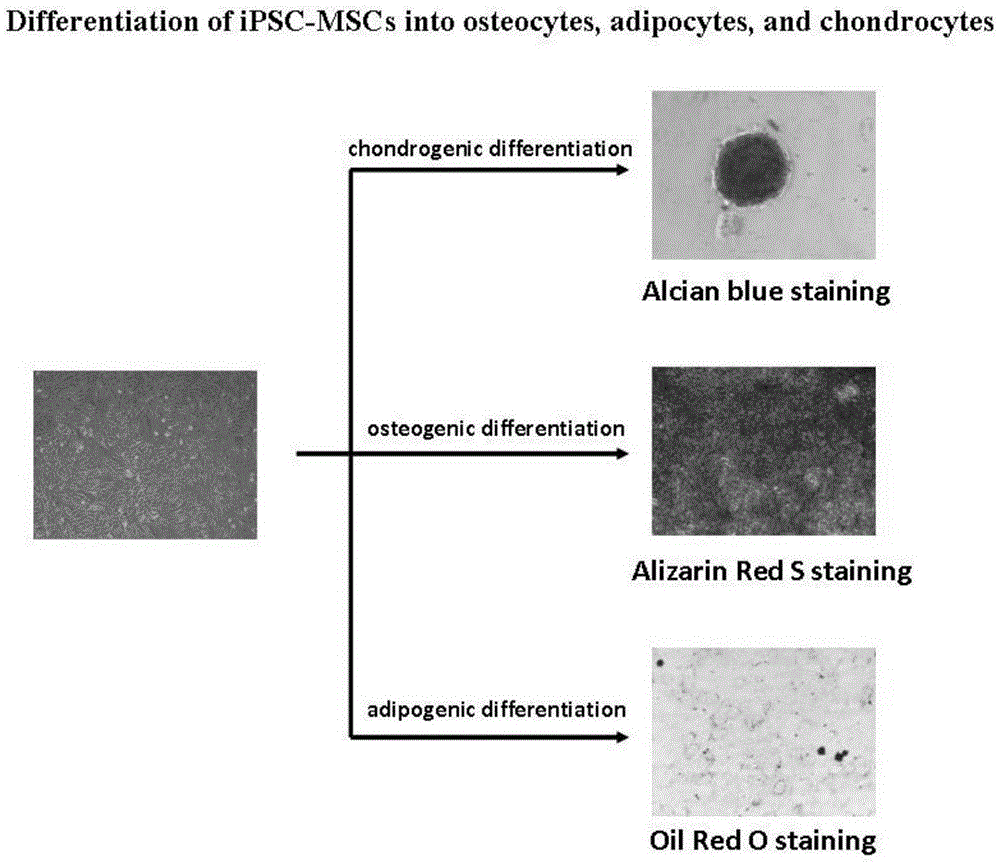
[0070] [Example 2] Optimizing the method for directed differentiation of iPS cells to mesenchymal stem cells
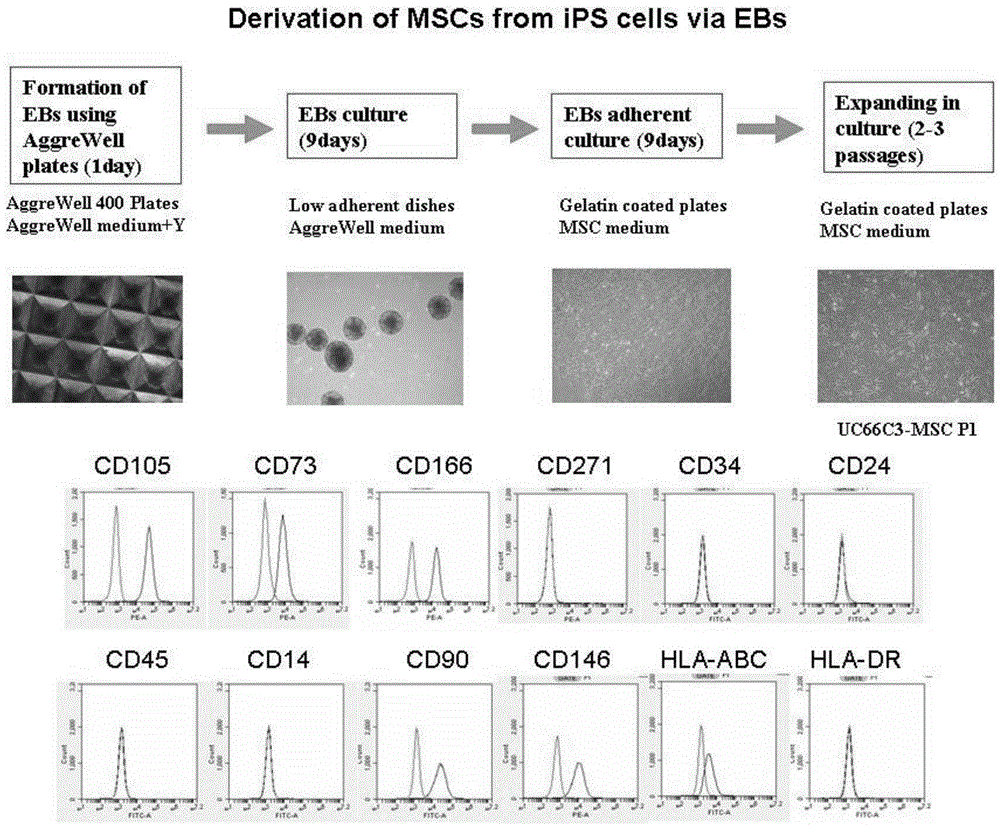
[0071] We optimized the method of directed differentiation of iPS cells to mesenchymal stem cells ( figure 2 ) includes: (1) iPS cells form embryoid bodies (EBs); (2) embryoid body suspension culture differentiates into mesoderm cells; (3) embryoid body adherent culture differentiated into mesoderm cells differentiates into mesenchymal stem cells .
[0072] We prepared embryoid bodies from iPS cells using AggreWell plates, which have microwells that can be used to aggregate iPS cells into embryoid bodies. When preparing embryoid bodies, add the single-cell suspension to the AggreWell culture plate, and then distribute the cells evenly in the microwells by centrifugation. After at least 24 hours of culture, the cells in each microwell are aggregated into clusters , prepared into embryoid bodies. The embryoid bodies obtained from AggreWell culture plates are very co...
Embodiment 3
[0073] [Example 3] A method of using urine cells to transdifferentiate into mesenchymal stem cells
[0074] This embodiment relates to a method for generating mesenchymal stem cells through transcription factor-mediated transdifferentiation of urine cells, which specifically includes the following steps ( Figure 5 ):
[0075] 1) Introduce non-integrated episomal vectors (pEP4EO2SEN2K: 3.0 μg, pEP4EO2SET2K: 3.2 μg, pCEP4-M2L: 2.4 μg) expressing transcriptional regulators OCT4, SOX2, NANOG, KLF4 and LIN28 into 1 million urine cells, and then The cells were divided into three 10cm dishes pre-coated with matrigel and cultured with fibroblast medium;
[0076] 2) On the second day after transfection, the fibroblast medium was replaced with N2B27 conditioned medium (containing MEK inhibitor PD0325901, GSK3b inhibitor CHIR99021, TGF-b / Activin / Nodalreceptor inhibitor A-83-01, ROCK inhibitor HA- 100and human leukemia inhibitory factor (LIF), replace fresh medium every 2 days;
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com