Fuel cell alloy catalyst preparation method
A technology for alloy catalysts and fuel cells, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc. Increased resistance and other problems, to achieve the effects of large-scale production, improved stability, and improved catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Add 10mL of deionized water to the beaker, then add 0.228gNi(NO 3 ) 2 ·6H 2 O or 0.226gCo(NO 3 ) 2 ·6H 2 O was added to the beaker and stirred evenly.
[0040] 2. Add 0.1g of 50wt% Pt / C to the above solution, and ultrasonicate for 30min until uniform.
[0041] 3. Add ammonia water diluted at a volume ratio of 1:4 to the above solution, adjust the pH=9, stir at room temperature for 3 hours, then centrifuge the above mixture, and finally dry it under vacuum at 60°C.
[0042] 4. The catalyst prepared above was heated at 800°C, 5% H 2 / Ar atmosphere thermal reduction treatment for 1 h, and then naturally cooled to room temperature.
[0043] 5. Mix the catalyst obtained in step 4 with 30mL, 1mol / L of H 2 SO 4 The solutions were mixed, sonicated for 30min, and then stirred in an oil bath at 80°C for 24h. Then it was centrifuged, washed several times, and finally dried under vacuum at 60°C.
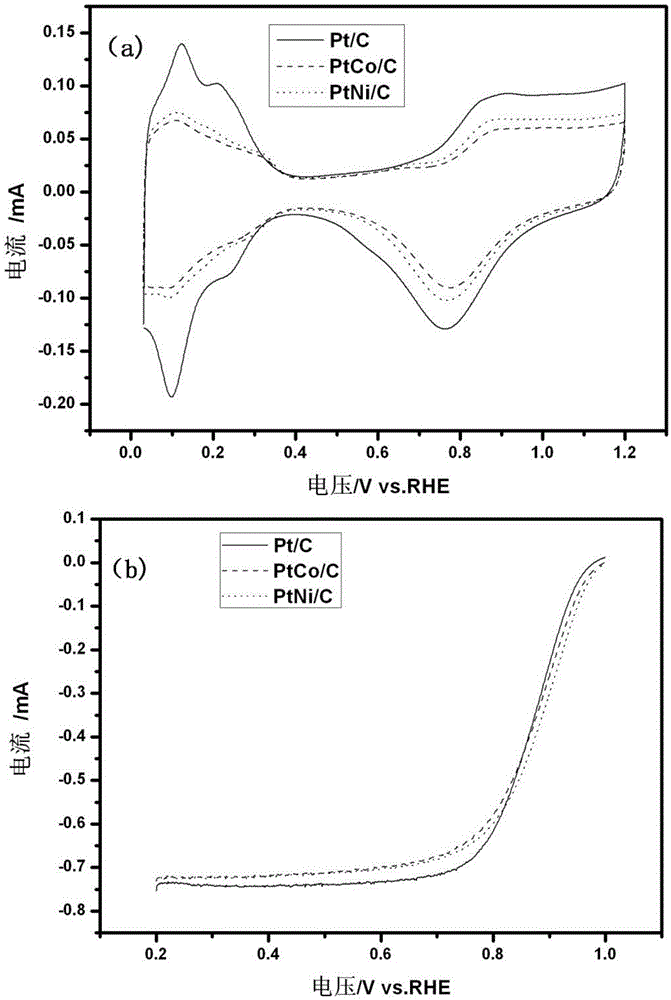
[0044] The cyclic voltammetry and oxygen reduction polarization curves ...
Embodiment 2
[0046] 1. Add 15mL of deionized water to the beaker, then add 0.193gNi(NO 3 ) 2 ·6H 2 O was added to the beaker and stirred evenly.
[0047] 2. Add 0.1g of 50wt% Pt to the above solution 3 Pd / C, sonicate for 30min until uniform.
[0048] 3. Add ammonia water diluted at a volume ratio of 1:4 to the above solution, adjust the pH to 12, stir at room temperature for 1 hour, then centrifuge the above mixture, and finally dry it under vacuum at 60°C.
[0049] 4. The catalyst prepared above was heated at 900°C under H 2 Thermal reduction treatment under atmosphere for 0.5h, then naturally cooled to room temperature.
[0050] 5. Mix the catalyst obtained in step 4 with 40 mL, 0.5 mol / L of H 2 SO 4 The solutions were mixed, sonicated for 30min, and then stirred in an oil bath at 60°C for 48h. Then it was centrifuged, washed several times, and finally dried under vacuum at 60°C.
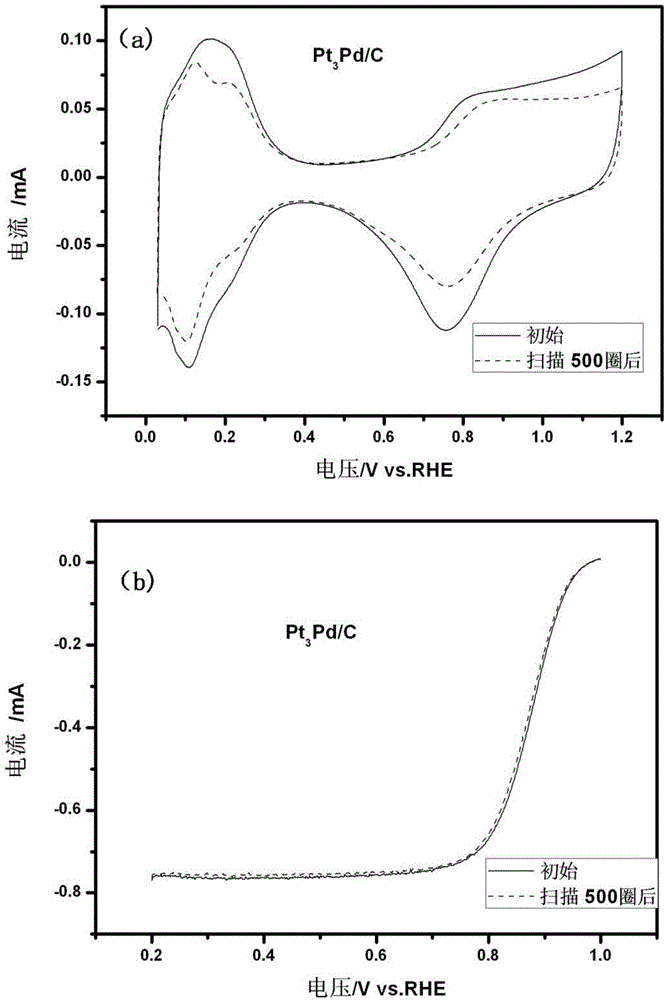
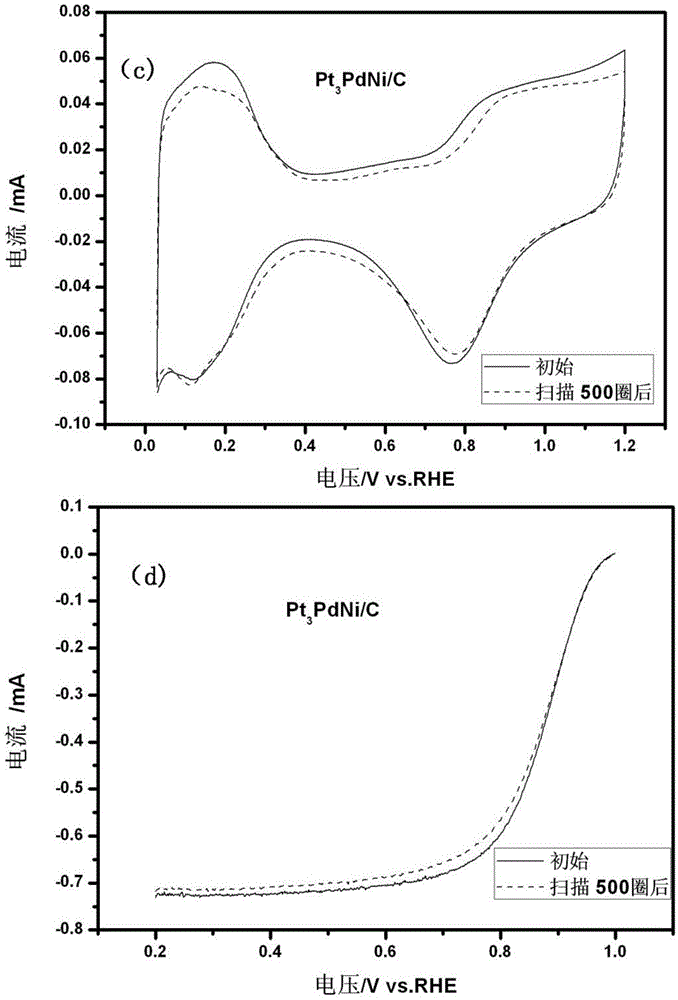
[0051] The activity of the catalyst that present embodiment makes and stability test curve are as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com