Application of triacetyl-3-hydroxyphenyl adenosine in preparing medicines for improving insulin resistance and related diseases
A technology of hydroxyphenyladenosine and insulin resistance, applied in the field of medicine, can solve problems such as difficulty in using diabetes drugs, liver dysfunction, weight gain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
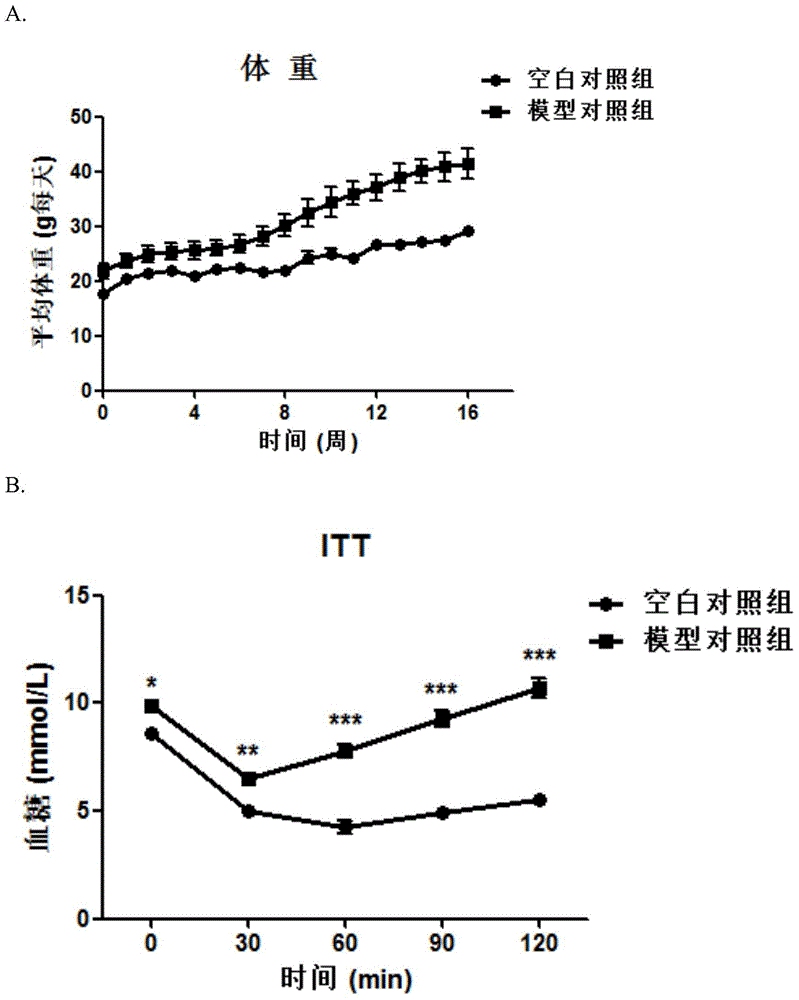
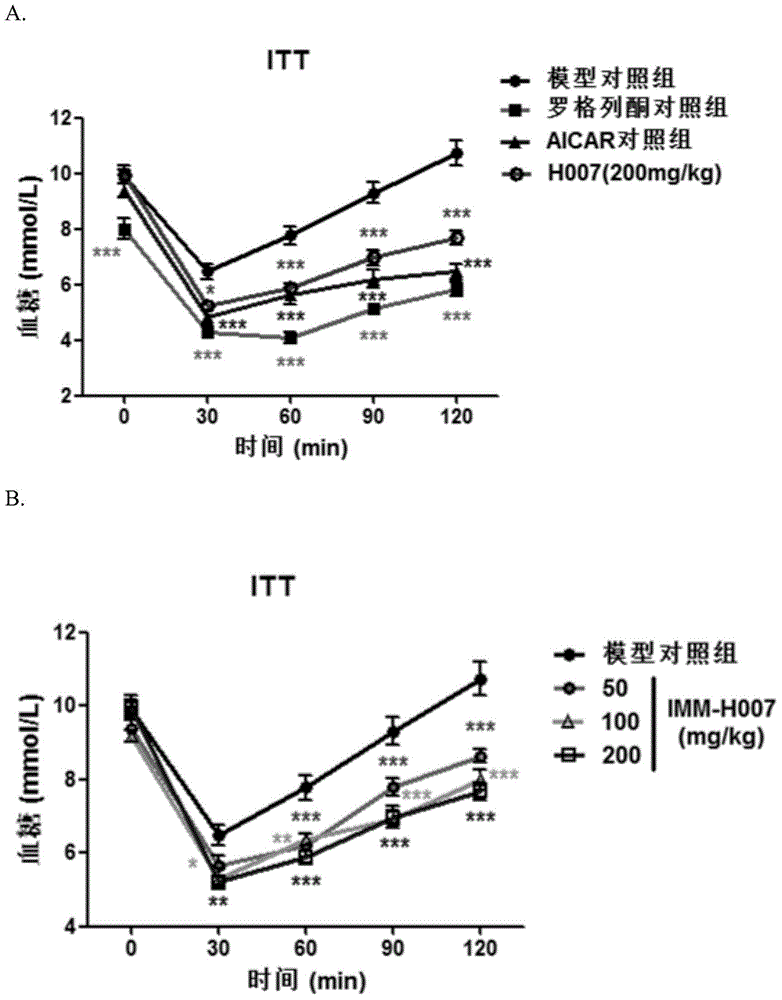
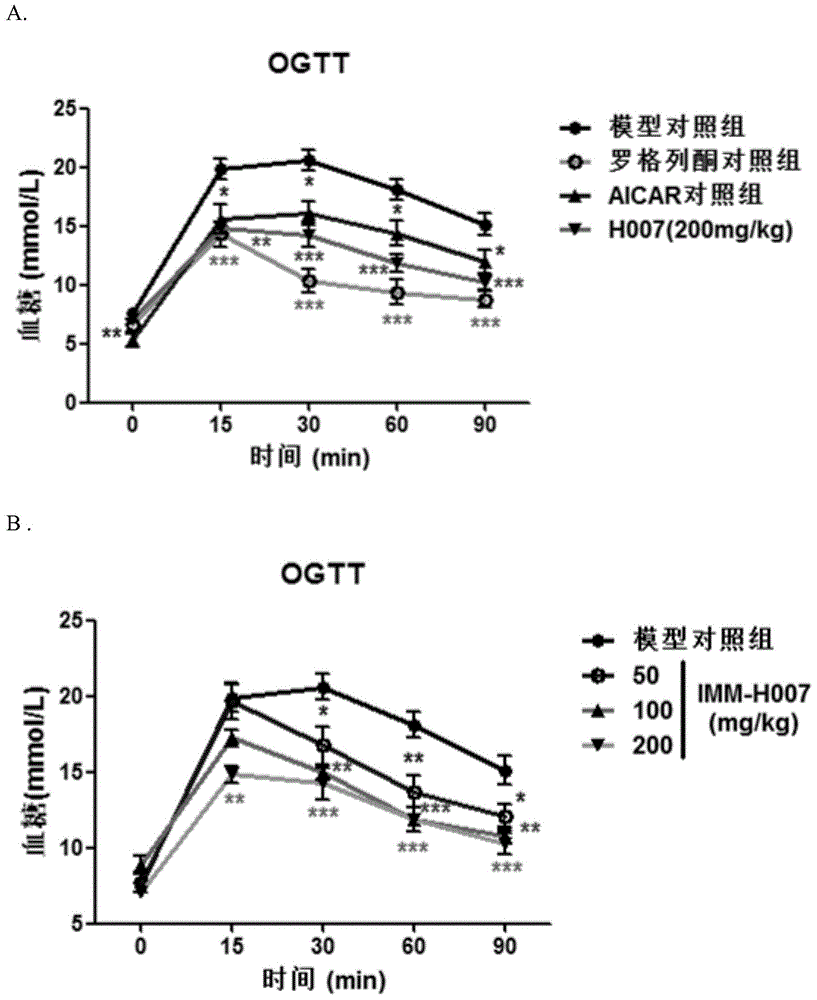
[0034] Example 1: Effect of Triacetyl-3-Hydroxyphenyl (IMM-H007) on Diet-Induced Insulin Resistance Mice
[0035] Materials and methods
[0036] 1.1 Animals and experimental design
[0037] C57BL / 6J mice at 8 weeks old were fed adaptively for 1 week, then fed with high-fat diet (ResearchDiets, D12451) for 16 weeks, and the successful modeling mice were screened by insulin resistance test (ITT), and randomly divided into 6 groups according to body weight , were model control group, positive control group (rosiglitazone control group 15mg / kg and AICAR control group 375mg / kg), IMM-H007 low-dose group (50mg / kg), IMM-H007 medium-dose group (100mg / kg kg) and IMM-H007 high-dose group (200mg / kg), 7 rats in each group, and a normal diet control group (6 rats). Oral administration once a day, normal control group and model group were given the same volume of vehicle (0.1ml / 10g body weight).
[0038] 1.2 Detection indicators
[0039]1) Insulin resistance experiment: Insulin resistanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com