Vermiculite surface in-situ grown potassium niobate material and preparation method thereof
An in-situ growth, potassium niobate technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve cost constraints, high recovery and recycling, The effective surface area and photocatalytic efficiency are weakened, so as to achieve the effect of improved photodegradation performance and high recycling cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The exfoliated vermiculite was soaked in ethanol for 12h, washed with deionized water and dried for later use. In a 250ml reactor, dissolve 39.2g KOH in 30ml deionized water, stir to dissolve completely until the solution is clear. Get 0.5g of treated vermiculite and place it in the above reaction vessel, then add 1.84gNbCl 5, stirred at room temperature for 30 minutes. After the stirring is completed, transfer the reaction liquid into an 80ml reaction kettle, and react at a constant temperature of 180°C for 12 hours. After the solution is cooled, take out the vermiculite, rinse it repeatedly with deionized water, and dry it at 60°C for 24 hours to obtain KNbO 3 / VMT.
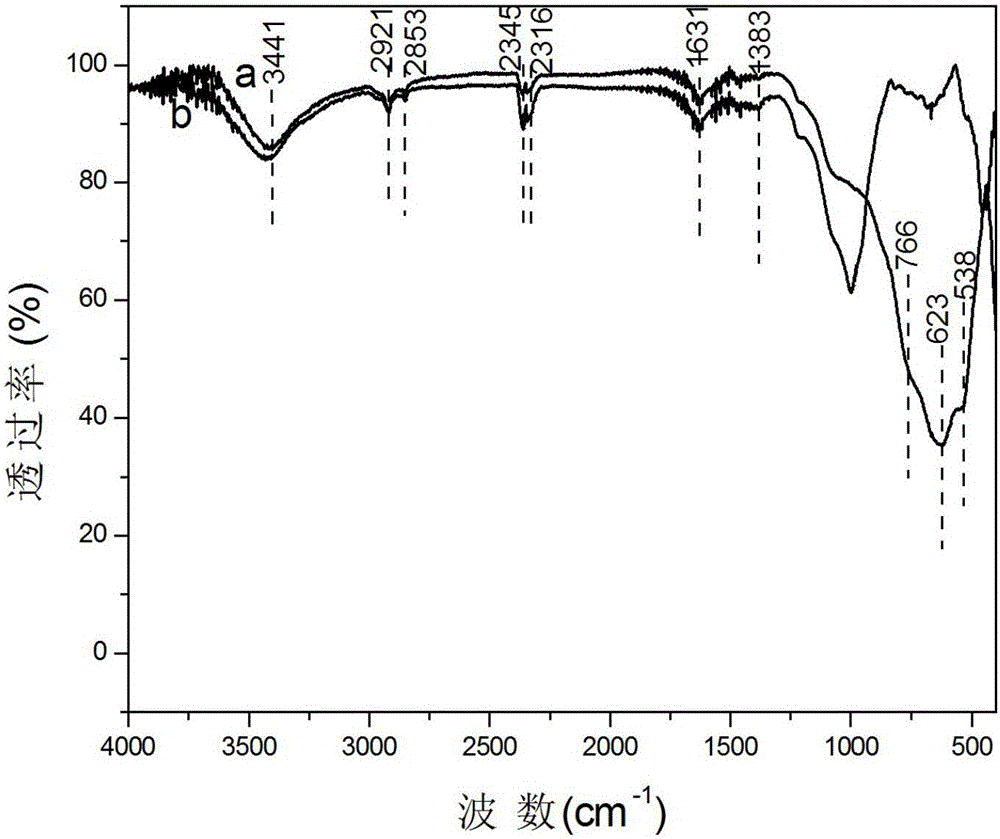
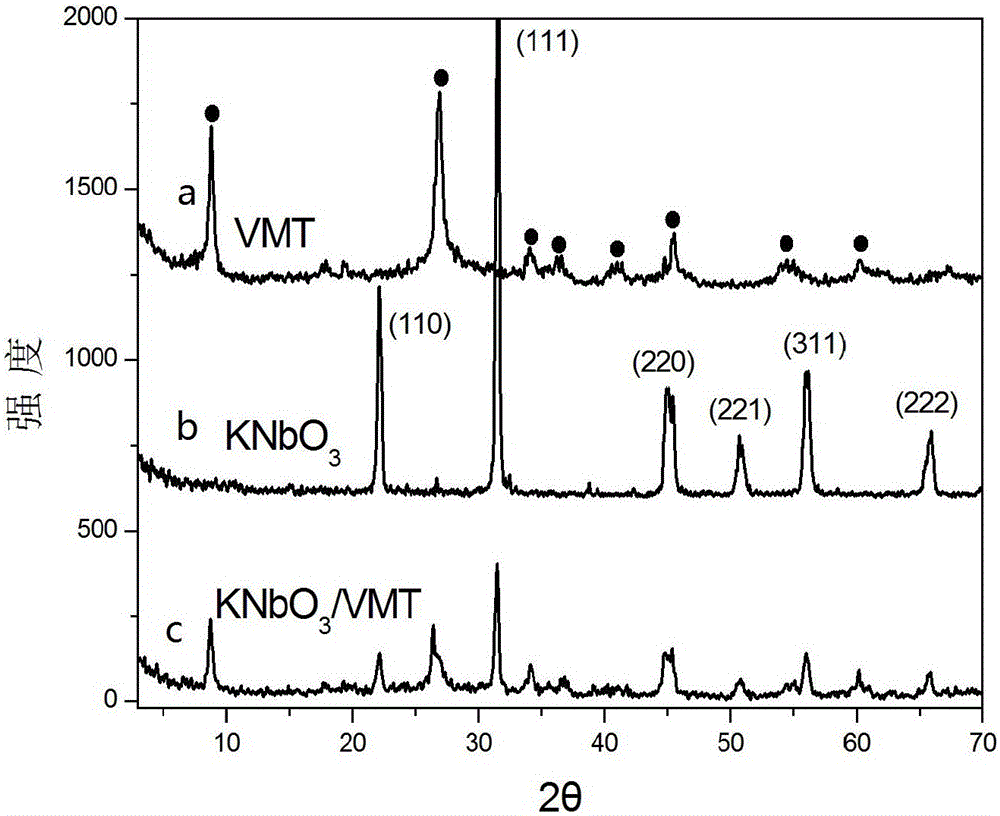
[0026] Characterization of its structure as Figure 1-5 As shown, it shows that the needle-like structure formed on the surface of vermiculite is potassium niobate.
Embodiment 2
[0028] The exfoliated vermiculite was soaked in ethanol for 12 hours, washed with deionized water and dried for later use. In a 250ml reactor, dissolve 39.2g KOH in 50ml deionized water, stir to dissolve completely until the solution is clear. Get 1g of treated vermiculite and place it in the above reaction vessel, then add 1.84gNbCl 5 , stirred at room temperature for 30 minutes. After the stirring is completed, transfer the reaction solution into an 80ml reaction kettle, and react at a constant temperature of 200°C for 18 hours. After the solution is cooled, take out the vermiculite, rinse it repeatedly with deionized water, and dry it at 60°C for 24 hours to obtain KNbO 3 / VMT.
Embodiment 3
[0030] The exfoliated vermiculite was soaked in ethanol for 12 hours, washed with deionized water and dried for later use. In a 250ml reactor, dissolve 39.2g KOH in 70ml deionized water, stir to dissolve completely until the solution is clear. Get 1.5g of treated vermiculite and place it in the above reaction vessel, then add 1.84gNbCl 5 , stirred at room temperature for 30 minutes. After the stirring is completed, transfer the reaction solution into a 100ml reaction kettle, and react at a constant temperature of 160°C for 24 hours. After the solution is cooled, take out the vermiculite, rinse it repeatedly with deionized water, and dry it at 60°C for 24 hours to obtain KNbO 3 / VMT.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com