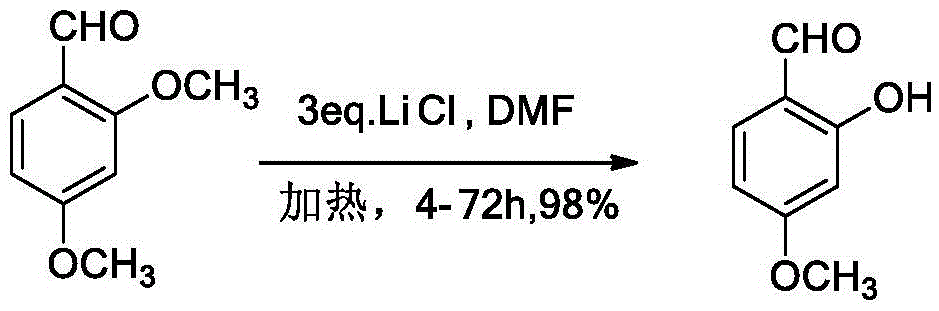
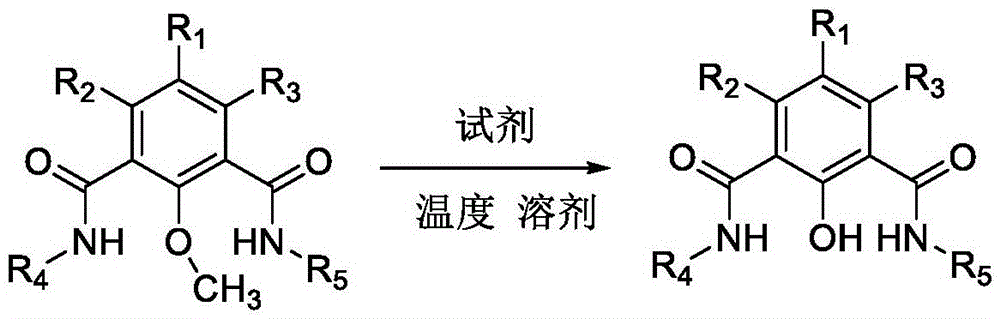
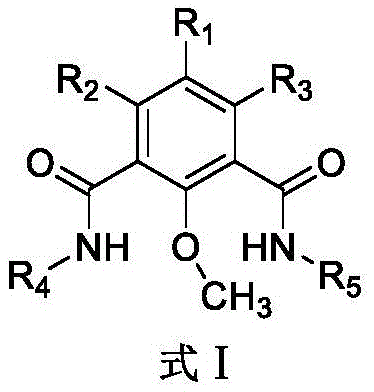
Method for removing methyl protecting group from phenolic hydroxyl under mild condition
A technology of phenolic hydroxyl methyl group and protecting group, applied in the field of organic synthesis, can solve the problems of difficult reaction process, difficult preservation of reagents, high price, etc., and achieve the effects of effective specific structure, mild conditions and few side reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]
[0015] 1mmol (0.22g) 2-methoxy-N 1 ,N 3 - Dimethylisophthalimide and 2mmol (0.64g) tetrabutylammonium bromide were placed in a 50ml round-bottomed flask, vacuumed, replaced with nitrogen, then added 10ml of dry tetrahydrofuran, stirred, and heated To 70 °C, TLC trace spot plate. After the reaction was completed, 1M hydrochloric acid was added to the reaction flask to quench. Add an appropriate amount of saturated saline and dichloromethane, extract and separate the liquids, combine the organic phases, dry over anhydrous sodium sulfate, and spin dry under reduced pressure. The residue is quickly separated by column chromatography to obtain 0.19 g of the product, with a yield of 91%.
Embodiment 2
[0017] 1mmol (0.22g) 2-methoxy-N 1 ,N 3 - Dissolve dimethylisophthalimide in tetrahydrofuran, then add tetrabutylammonium bromide, heat and stir, and react at 70 degrees Celsius for 10 hours. After the reaction, add 1M hydrochloric acid to the reaction bottle to quench . Add an appropriate amount of saturated saline and dichloromethane, extract and separate the liquids, combine the organic phases, dry with anhydrous sodium sulfate, and spin dry under reduced pressure. The residue is quickly separated by column chromatography to obtain 0.185 g of the product, with a yield of 89%.
Embodiment 3
[0019] 1mmol (0.22g) 2-methoxy-N 1 ,N 3 - Dissolve dimethylisophthalimide in 10ml tetrahydrofuran, then add 2mmol (0.64g) tetrabutylammonium bromide, heat and stir, and react at 70 degrees Celsius for 10 hours. Add 1M hydrochloric acid to quench. Add an appropriate amount of saturated saline and dichloromethane, extract and separate the liquids, combine the organic phases, dry over anhydrous sodium sulfate, and spin dry under reduced pressure. The residue is quickly separated by column chromatography to obtain 0.185 g of the product, with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com