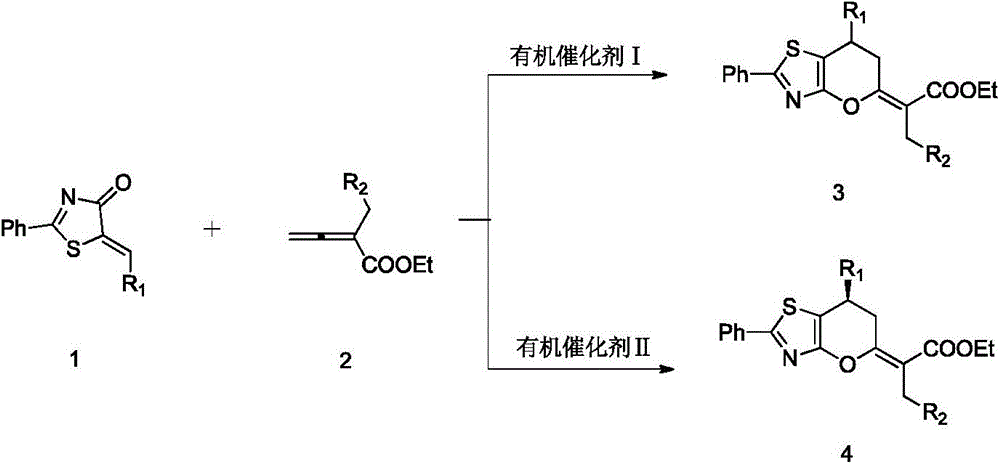
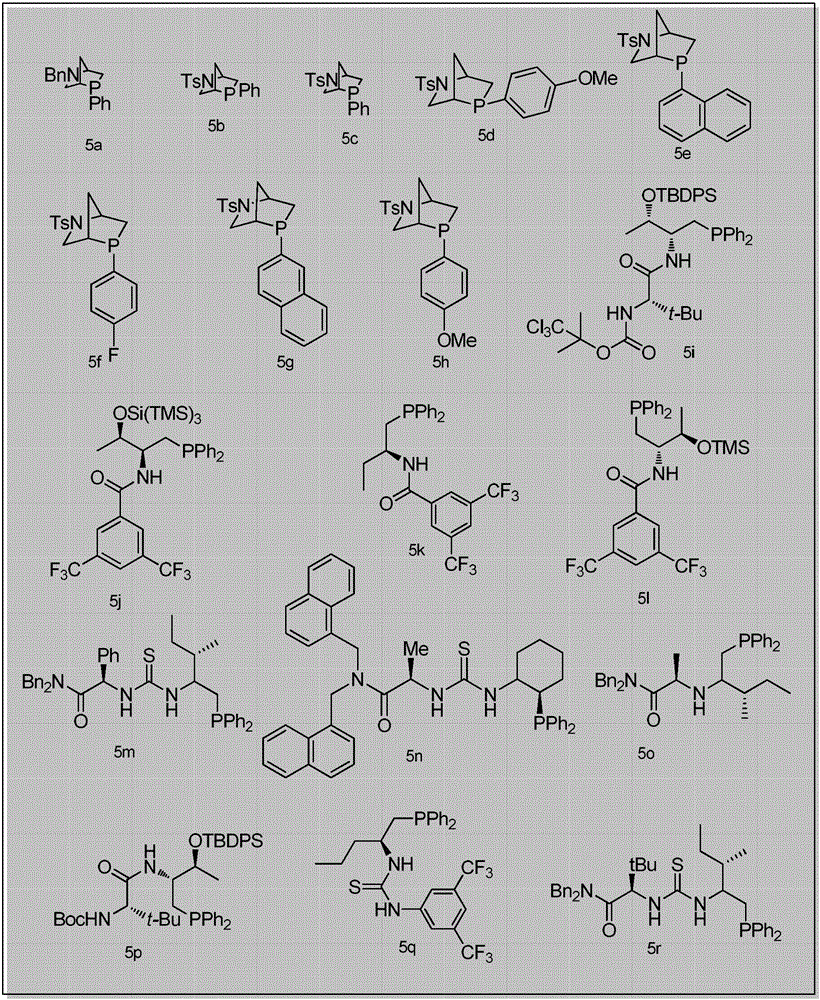
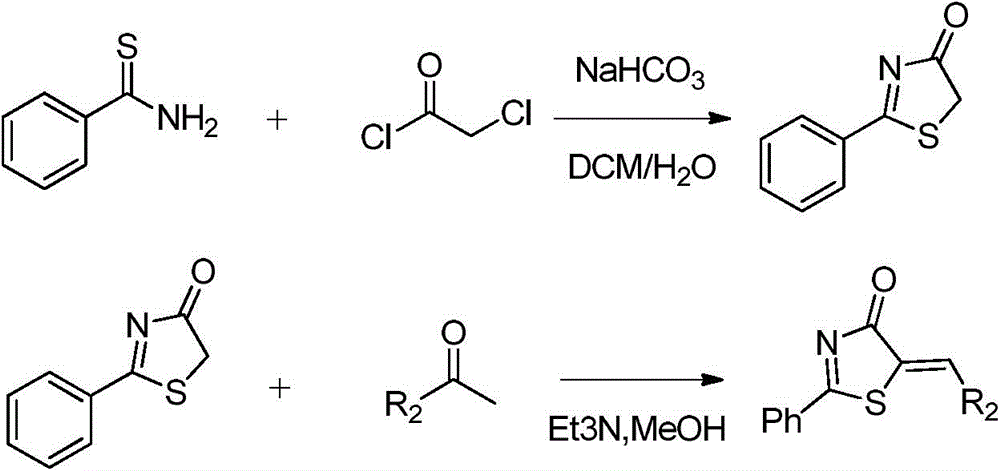
2,3-dihydropyranothiazole derivatives and preparation method thereof
A technology for dihydropyranothiazole derivatives and derivatives, which is applied in the field of pyranothiazole derivatives and their preparation, can solve problems such as unreported methods, and achieve increased diversity, high stereoselectivity and enantiometry selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] With 0.0265g (0.100mmol) compound 1a, 0.0303g (0.150mmol) compound 2a, Put MS100mg and 1mL toluene into a dry 15mL Shrek tube, add 2.00mg (0.010mmol) methyl diphenylphosphine and mix well to carry out cycloaddition reaction. In this reaction system, the molar ratio of compound 1a to compound 2a is 1: 1.5, and organophosphorus catalyst I accounts for 10% of the molar percentage of compound 1a, and is stirred at 40° C. for 2 hours, and concentrated with a rotary evaporator to pass through the column (acetic acid Ethyl ester:petroleum ether=1:20, v / v), to obtain 35.0 mg of product 3aa, with a yield of 75%.
Embodiment 2
[0029]
[0030] With 0.0265g (0.100mmol) compound 1a, 0.0303g (0.150mmol) compound 2a, Put MS100mg and 1mL toluene into a dry 15mL Shrek tube, add 1.39mg (0.010mmol) dimethylphenylphosphine and mix well to carry out cycloaddition reaction. In this reaction system, the molar ratio of compound 1a to compound 2a is 1: 1.5, and organophosphorus catalyst I accounts for 10% of the molar percentage of compound 1a, and is stirred at 40° C. for 2 hours, and concentrated with a rotary evaporator to pass through the column (acetic acid Ethyl ester:petroleum ether=1:20, v / v), to obtain 37.4 mg of product 3aa, with a yield of 80%.
Embodiment 3
[0032]
[0033] With 0.0265g (0.100mmol) compound 1a, 0.0303g (0.150mmol) compound 2a, Put MS100mg and 1mL toluene into a dry 15mL Shrek tube, add 2.10mg (0.010mmol) tributylphosphine and mix well for cycloaddition reaction. In this reaction system, the molar ratio of compound 1a to compound 2a is 1: 1.5, and organophosphorus catalyst I accounts for 10% of the molar percentage of compound 1a, and is stirred at 40° C. for 2 hours, and concentrated with a rotary evaporator to pass through the column (acetic acid Ethyl ester:petroleum ether=1:20, v / v), to obtain 21.0mg of product 3aa, yield 45%.
[0034] It can be seen from the above that the effect is very good when dimethylphenylphosphine is selected as the catalyst for the synthesis of compound 3. The 3aa NMR data are as follows:
[0035] 1 HNMR (300MHz, CDCl 3 )δ7.96-7.86 (m, 2H), 7.48-7.40 (m, 3H), 7.35-7.29 (m, 5H), 7.28-7.15 (m, 5H), 4.38-4.28 (q, J=7.1Hz, 1H), 4.14-4.04(m, 2H), 3.97(q, J=7.1Hz, 2H), 3.86-3.76(m, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com